Lithium-ion intercalation by coupled ion-electron transfer
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
The underlying reaction mechanism in lithium-ion batteries remains poorly understood. We provide experimental and theoretical evidence that lithium intercalation occurs by coupled ion-electron transfer, where ion transfer across the electrode-electrolyte interface is facilitated by electron transfer to a neighboring redox site. Electrochemical measurements for a variety of common electrode and electrolyte materials reveal a universal dependence of the (de-)intercalation rate on Li+ vacancy fraction, as well as temperature and electrolyte effects consistent with the theory, which could be used to guide the molecular design of lithium-ion battery interfaces.
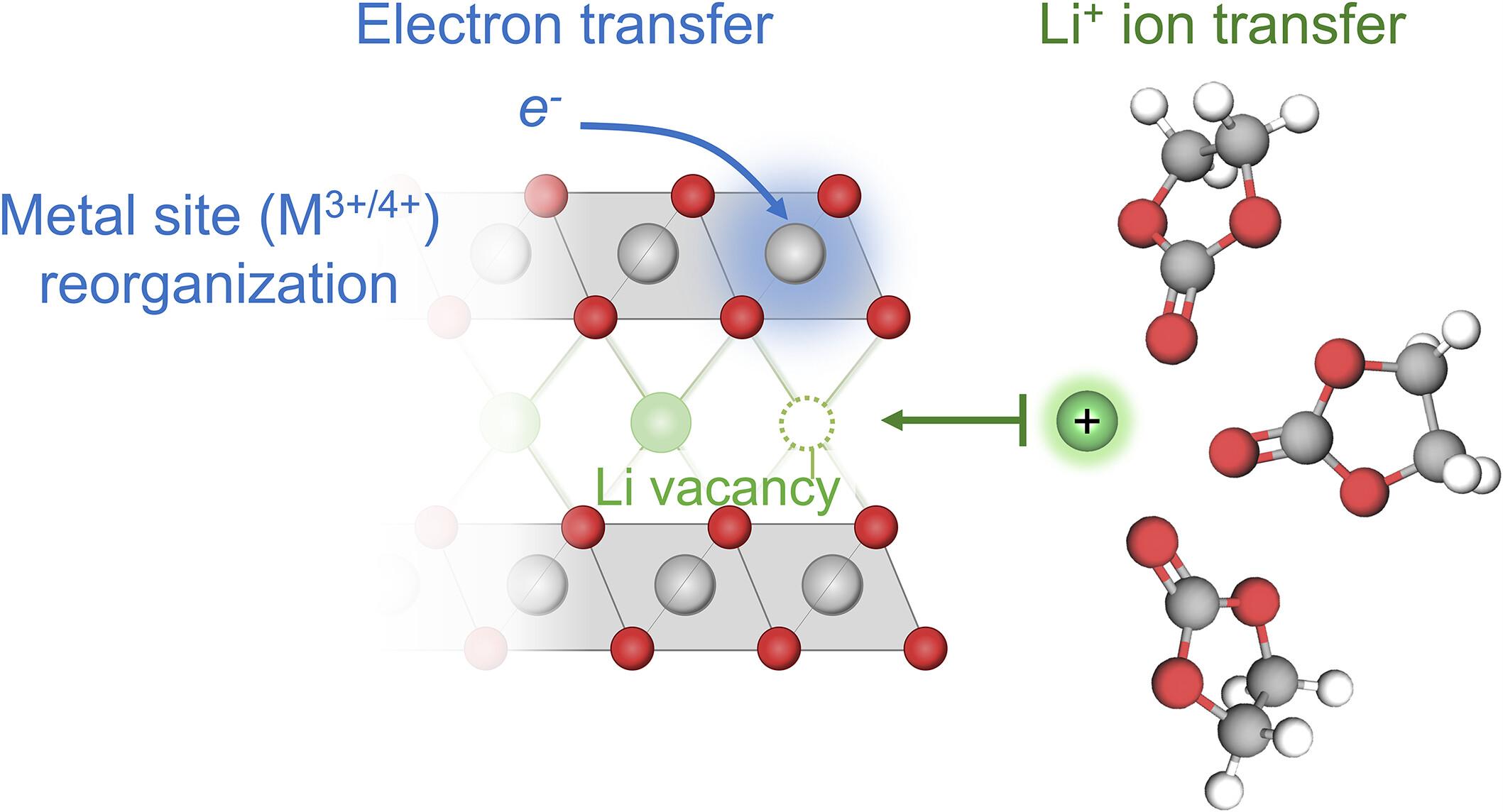
离子-电子耦合转移的锂离子嵌入
锂离子电池的潜在反应机制仍然知之甚少。我们提供了实验和理论证据,证明锂嵌入是通过离子-电子耦合转移发生的,其中电子转移到邻近的氧化还原位点促进了离子在电极-电解质界面上的转移。对多种常用电极和电解质材料的电化学测量表明,(脱)插层速率普遍依赖于Li +空位分数,以及温度和电解质效应,这与理论一致,可用于指导锂离子电池界面的分子设计。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


