Mitigation or promotion: The role of phenols and plant extracts in acrylamide formation
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The effects of different phenols (10−4−1 mmol) on acrylamide were evaluated in two Maillard reaction model systems (low-moisture and aqueous). High additions of most phenols decreased acrylamide, while tyrosol did not. Low additions rather increased acrylamide levels, particularly under low-moisture conditions. A pH-focused model revealed that some phenols lowered acrylamide levels in addition to pH and that the initial pH strongly influenced their impact: at pH 5, ellagic acid and ferulic acid lowered acrylamide levels in addition to pH, while at pH 8, the effect was diminished or even reversed for ferulic acid. Oxidation of phenols and their degradation products could account for these observations. Moreover, commercial plant extracts were evaluated for acrylamide mitigation. All extracts were also sources for acrylamide formation despite being a source of phenolic compounds. Nonetheless, green tea extract reduced acrylamide levels. Altogether, different factors have to be considered for acrylamide mitigation with phenolic ingredients.
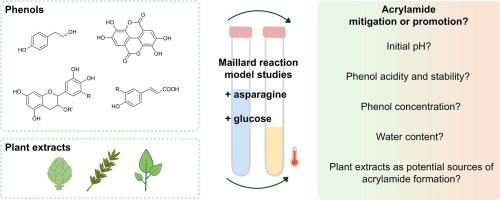
缓解或促进:酚类和植物提取物在丙烯酰胺形成中的作用
在两种美拉德反应模型体系(低湿和水)中考察了不同酚(10−4−1 mmol)对丙烯酰胺的影响。大多数酚类的高添加量降低了丙烯酰胺,而酪醇则没有。低添加量反而增加丙烯酰胺含量,特别是在低水分条件下。一个以pH为中心的模型显示,一些酚类降低了丙烯酰胺的水平,而不是pH值,并且初始pH值强烈影响它们的作用:在pH值 5时,鞣花酸和阿魏酸降低了丙烯酰胺的水平,而在pH值 8时,阿魏酸的效果减弱甚至逆转。苯酚的氧化及其降解产物可以解释这些观察结果。此外,还评估了商业植物提取物对丙烯酰胺的缓解作用。所有提取物也是丙烯酰胺形成的来源,尽管是酚类化合物的来源。尽管如此,绿茶提取物降低了丙烯酰胺的含量。总之,用酚类成分减少丙烯酰胺必须考虑不同的因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


