Uronium from X-ray-Desorbed Urea Enables Sustainable Ultrasensitive Detection of Amines and Semivolatiles
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
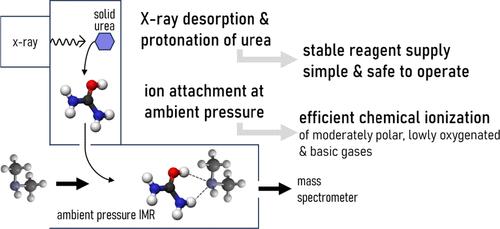
Comprehensive mass spectrometric detection requires multiple ionization schemes. Chemical ionization (CI) at a low pressure is suitable for the detection of weakly polar volatile organic compounds (VOCs). Negative-mode ionization at ambient pressure delivers a superior performance for polar acidic compounds. Positive-mode CI has been explored to detect basic and polar neutral compounds for which negative polarity and low-pressure ionization techniques have shown insufficient performance. Several ion attachment reagents have been proposed for sensitive and soft ionization. These reagents are often reactive, toxic, and difficult to control, which impede their applicability and operability. Inspired by these challenges, we explored uronium, protonated urea, as an alternative for ionizing moderately oxygenated, basic, and polar neutral compounds at ambient pressure. Urea, a nontoxic solid with negligible vapor pressure, is desorbed by X-ray irradiation, forming the uronium ion. We experimentally determined the behavior of uronium ionization under different humidities for several semivolatile organic compounds (SVOCs), amines, and ammonia and explored the mechanism using theory. In laboratory measurements of α-pinene and dimethyl sulfide (DMS) oxidation systems, we characterized how uronium complements other ionization schemes. Excellent sensitivities were achieved for several key components (including amines, dimethyl sulfoxide (DMSO), N-methyl-2-pyrrolidone, verbenone, and dimethylformamide (DMF)), requiring sample sizes of only a few attomoles for detection in individual spectra, equivalent to detection limits at the low to mid parts per quadrillion by volume (ppqv) level. Uronium exhibits a tendency to selectively form strong ion–molecular clusters, which renders the ionization robust against sample humidity changes. X-ray desorption of solid urea simplifies reagent supply handling and ensures the long-term stability of the ion production system, providing a safe and sustainable alternative to equivalent CI methods.
从x射线解吸尿素中提取的尿素能够持续超灵敏地检测胺和半挥发性物质
全面的质谱检测需要多种电离方案。低压化学电离法(CI)适用于弱极性挥发性有机物(VOCs)的检测。环境压力下的负模式电离为极性酸性化合物提供了优越的性能。正模式CI已被用于检测碱性和极性中性化合物,而负极性和低压电离技术已显示出不足的性能。已经提出了几种离子附着试剂用于敏感和软电离。这些试剂往往反应性强、毒性大、难以控制,阻碍了它们的适用性和可操作性。受到这些挑战的启发,我们探索了尿素,即质子化尿素,作为在环境压力下电离中等氧合、碱性和极性中性化合物的替代品。尿素是一种无毒的固体,蒸气压可以忽略不计,通过x射线照射解吸,形成铀离子。我们实验测定了几种半挥发性有机化合物(SVOCs)、胺和氨在不同湿度下的铀离子电离行为,并用理论探讨了其机理。在α-蒎烯和二甲基硫醚(DMS)氧化系统的实验室测量中,我们表征了铀是如何补充其他电离方案的。对几种关键成分(包括胺、二甲亚砜(DMSO)、n -甲基-2-吡咯烷酮、马鞭草酮和二甲基甲酰胺(DMF))实现了极好的灵敏度,在单个光谱中仅需几个原子的样本量即可检测,相当于每千万亿分之一体积(ppqv)水平的中低检测限。铀元素表现出选择性形成强离子分子簇的倾向,这使得电离对样品湿度变化具有很强的鲁棒性。固体尿素的x射线解吸简化了试剂供应处理,确保了离子生产系统的长期稳定性,为等效CI方法提供了一种安全和可持续的替代方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


