Study on the effects of representative spice polyphenols on the in vitro digestive properties of tilapia myofibrillar protein and its mechanism of action: based on physical and chemical properties and enzyme activity
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Food flavorings are essential components in processing due to their safety, efficacy, and additional flavour and nutritional benefits. To investigate the effects of polyphenolic compounds in flavorings on protein digestibility, this study selected five high-concentration phenolic compounds (quercetin, 8-gingerol, naringin, apigenin, and ferulic acid) from flavorings to prepare protein-polyphenol complexes. Simulated gastrointestinal digestion results showed that protein digestibility was significantly reduced after polyphenol binding; SEM and AFM revealed that protein structure was disrupted by polyphenol addition; FT-IR confirmed changes in secondary structure, particularly a reduction in α-helix content. Fluorescence spectroscopy, molecular docking, and molecular dynamics results indicated that polyphenols bind to proteins via non-covalent bonds, exhibiting quenching effects on fluorescent amino acid residues in proteins; two-dimensional and three-dimensional spectroscopy and molecular docking confirmed that the five phenolic compounds bind to digestive enzymes via hydrogen bonds and hydrophobic interactions, causing static fluorescence quenching of digestive enzymes and reducing their digestive activity. This study provides insights into the interaction mechanisms between plant-derived polyphenols, tilapia protein, and proteases at the physicochemical and enzymatic activity levels, which may help expand the application of tilapia protein-polyphenol complexes in the food industry and provide a basis for the development and optimization of tilapia processed foods.
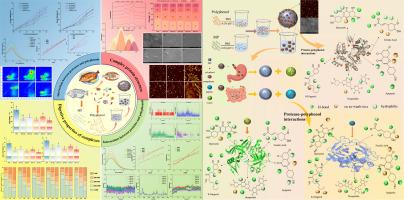
代表性香料多酚对罗非鱼肌纤维蛋白体外消化性能的影响及其作用机制研究:基于理化性质和酶活性
食品调味料是加工过程中必不可少的成分,因为它们具有安全性、有效性、额外的风味和营养价值。为了研究调味料中多酚类化合物对蛋白质消化率的影响,本研究从调味料中选取5种高浓度酚类化合物(槲皮素、8-姜辣素、柚皮素、芹菜素和阿魏酸)制备蛋白质-多酚复合物。模拟胃肠道消化结果显示,多酚结合后蛋白质消化率显著降低;SEM和AFM分析表明,多酚的加入破坏了蛋白质结构;FT-IR证实了二级结构的变化,特别是α-螺旋含量的降低。荧光光谱、分子对接和分子动力学结果表明,多酚通过非共价键与蛋白质结合,对蛋白质中的荧光氨基酸残基具有猝灭作用;二维、三维光谱和分子对接证实,5种酚类化合物通过氢键和疏水相互作用与消化酶结合,导致消化酶的静态荧光猝灭,降低消化酶的消化活性。本研究揭示了植物源性多酚、罗非鱼蛋白和蛋白酶在理化和酶活性水平上的相互作用机制,有助于扩大罗非鱼蛋白-多酚复合物在食品工业中的应用,并为罗非鱼加工食品的开发和优化提供依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


