7,8-Dihydroxyflavone attenuates cisplatin-induced cardiomyocyte apoptosis and mitochondrial dysfunction via the p53/Nrf2 pathway
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Cisplatin (CDDP), while effective as a chemotherapeutic agent, poses significant cardiovascular risks that constrain its clinical utility. This study investigated the cardioprotective effects of 7,8-dihydroxyflavone (7,8-DHF) against CDDP-induced toxicity and explored the underlying molecular mechanisms in cardiomyocytes. CDDP exposure produced dose-dependent cytotoxic effects, characterized by reduced cell viability and elevated lactate dehydrogenase (LDH) release. Co-treatment with 7,8-DHF markedly attenuated CDDP-induced cellular damage by preventing cell death, minimizing LDH leakage, and preserving mitochondrial membrane potential (MMP). The compound also suppressed cardiomyocyte apoptosis, evidenced by fewer TUNEL-positive cells and restoration of the Bcl-2/Bax ratio. 7,8-DHF decreased mitochondrial reactive oxygen species (ROS) accumulation and enhanced cellular antioxidant defenses by upregulating nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) signaling. Additionally, 7,8-DHF treatment decreased both 53BP1 foci formation and p53 protein expression. The specificity of these protective mechanisms was confirmed using pharmacological agents: Nutlin-3a (p53 activator) and Brusatol (Nrf2 inhibitor), both reversed the cardioprotective effects of 7,8-DHF, establishing the critical role of p53/Nrf2 pathway modulation. In summary, these findings demonstrate that 7,8-DHF protects against CDDP-induced cardiotoxicity by preserving mitochondrial function and preventing apoptosis through targeted inhibition of the p53 signaling and activation of Nrf2-mediated antioxidant responses in cardiomyocytes. Our study provides preliminary evidence for the potential of 7,8-DHF in mitigating CDDP-associated cardiac injury.
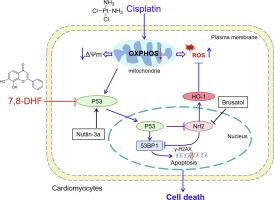
7,8-二羟黄酮通过p53/Nrf2途径减轻顺铂诱导的心肌细胞凋亡和线粒体功能障碍。
顺铂(CDDP)作为一种有效的化疗药物,具有显著的心血管风险,限制了其临床应用。本研究探讨了7,8-二羟黄酮(7,8- dhf)对cddp毒性的心脏保护作用,并探讨了其在心肌细胞中的潜在分子机制。CDDP暴露产生剂量依赖性的细胞毒性作用,其特征是细胞活力降低和乳酸脱氢酶(LDH)释放升高。与7,8- dhf共处理可通过防止细胞死亡、减少LDH泄漏和保留线粒体膜电位(MMP)显著减轻cddp诱导的细胞损伤。该化合物还抑制心肌细胞凋亡,通过减少tunel阳性细胞和恢复Bcl-2/Bax比率证明。7,8- dhf通过上调核因子-红细胞2相关因子2 (Nrf2)和血红素加氧酶-1 (HO-1)信号通路,降低线粒体活性氧(ROS)积累,增强细胞抗氧化防御能力。此外,7,8- dhf处理降低了53BP1灶的形成和p53蛋白的表达。这些保护机制的特异性被药理药物证实:Nutlin-3a (p53激活剂)和Brusatol (Nrf2抑制剂)都逆转了7,8- dhf的心脏保护作用,确立了p53/Nrf2通路调节的关键作用。总之,这些研究结果表明,7,8- dhf通过靶向抑制p53信号传导和激活nrf2介导的心肌细胞抗氧化反应,保护线粒体功能和防止细胞凋亡,从而保护心肌细胞免受cddp诱导的心脏毒性。我们的研究为7,8- dhf减轻cdp相关心脏损伤的潜力提供了初步证据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


