Dynamic U2AF cycling defines two phases of cotranscriptional pre-mRNA splicing
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Distinguishing functional splice sites from abundant cryptic sites in precursor messenger RNAs (pre-mRNAs) represents a fundamental challenge in decoding mammalian genomes. We demonstrate that the specific RNA polymerase II (Pol II) subunit RPB9 directly interacts with the 3′ AG dinucleotide binding factor U2AF1 to initiate 3′ splice site recognition. Combined with recent structural insights into Pol II–mediated 5′ splice site selection, these findings support a cotranscriptional mechanism to recognize paired 3′ and 5′ splice sites across individual exons. These initial exon definition events facilitate the recruitment of U2AF2 to heterodimerize with U2AF1, which also triggers U2AF1 release from elongating Pol II. Collectively, these results reveal dynamic U2AF cycling that partitions Pol II subunit–facilitated splice site recognition and subsequent Pol II–independent spliceosome assembly steps during cotranscriptional splicing.
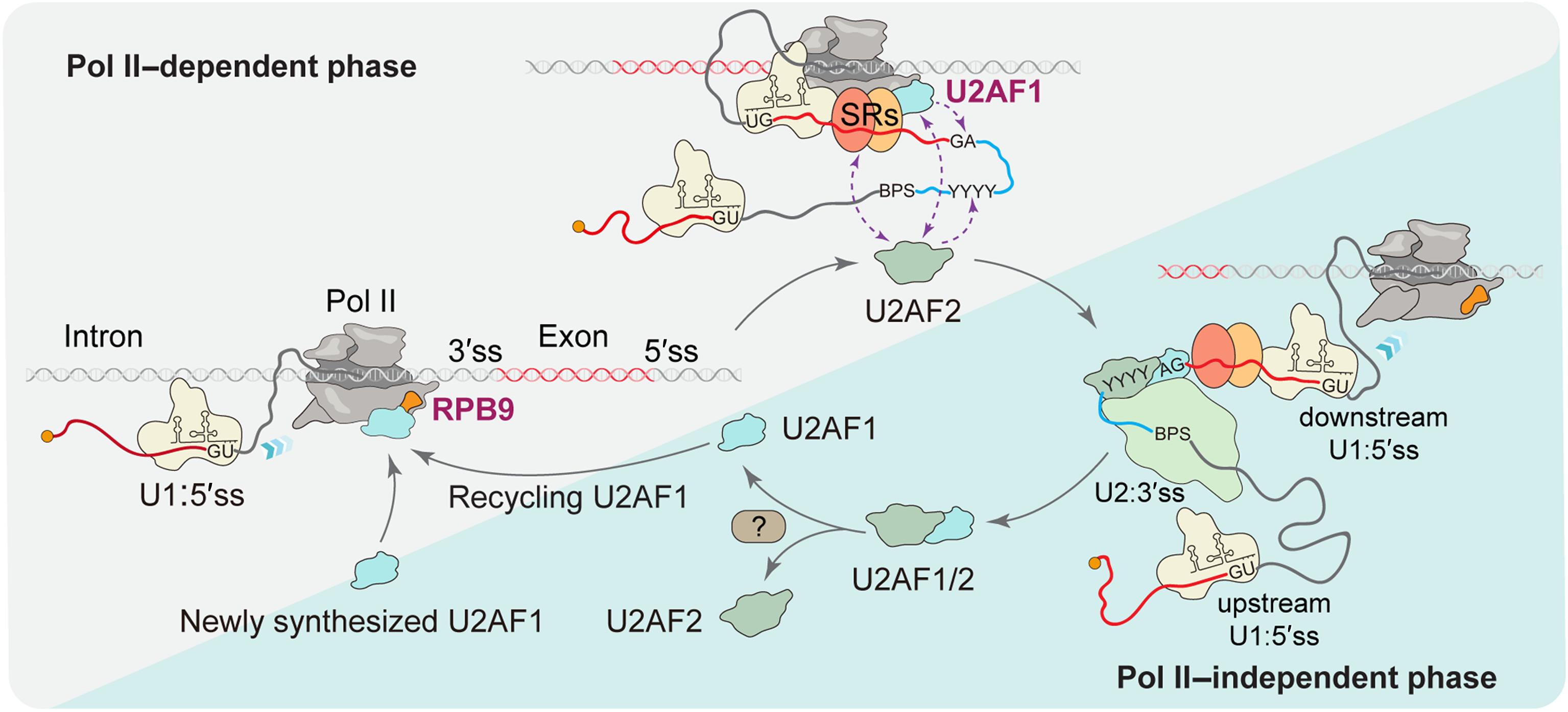
动态U2AF循环定义了共转录pre-mRNA剪接的两个阶段
区分前体信使rna (pre- mrna)的功能剪接位点和丰富的隐式位点是解码哺乳动物基因组的一个基本挑战。我们证明了特异性RNA聚合酶II (Pol II)亚基RPB9直接与3 ‘ AG二核苷酸结合因子U2AF1相互作用以启动3 ’剪接位点识别。结合最近对Pol ii介导的5 ‘剪接位点选择的结构见解,这些发现支持了一种识别单个外显子中配对的3 ’和5 '剪接位点的共转录机制。这些初始外显子定义事件促进了U2AF2与U2AF1的异二聚化,这也触发了U2AF1从延长Pol II中释放。总的来说,这些结果揭示了在共转录剪接过程中,动态U2AF循环分割了Pol II亚基促进的剪接位点识别和随后的Pol II非依赖性剪接体组装步骤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


