Unveiling the Structural and Biochemical Characteristics of an Acidophilic β-Mannanase from Soybean (Glycine max)
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
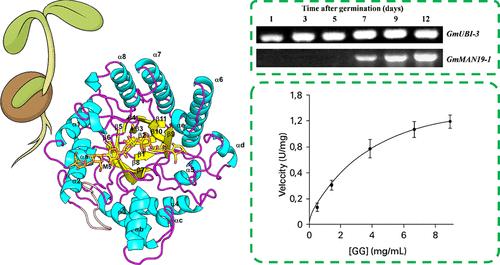
Endo-1,4-β-mannanase (EC 3.2.1.78) cleaves β-1,4-mannans in cell walls, facilitating endosperm softening and seed germination. Here, we present the structural and biochemical characterization of GmMAN19-1, a GH5_7 β-mannanase from soybean (Glycine max), a crop with substantial agricultural importance. GmMAN19-1 is specifically expressed in cotyledons during postgermination and exhibits acidophilic activity optimal at pH 4.6 and 40 °C. Crystal structures of GmMAN19-1 were determined at 1.39 and 2.62 Å in the apo form and in complex with mannopentaose (M5), respectively. The structure adopts a canonical (α/β)8 TIM barrel fold with a V-shaped active site groove. Notably, two distinct M5 binding modes were identified, suggesting dual functionality involving hydrolytic and transglycosylation activities. Site-directed mutagenesis further validated key catalytic and substrate-interacting residues: E186A abolished enzymatic activity, while Q267W altered transglycosylation product profiles and enhanced activity toward branched substrates. The binding groove can accommodate galactose side chains, supporting partial activity toward galactomannans. These findings provide comprehensive insights into the substrate specificity and catalytic mechanism of plant β-mannanases and establish GmMAN19-1 as a potential candidate for applications in food processing, biomass conversion, and industrial biotechnology, particularly due to its acidophilic nature and enhanced activity toward branched mannans.
大豆亲酸性β-甘露聚糖酶(Glycine max)的结构及生化特性研究
ender -1,4-β-甘露聚糖酶(EC 3.2.1.78)在细胞壁上裂解β-1,4-甘露聚糖,促进胚乳软化和种子萌发。在这里,我们介绍了GmMAN19-1的结构和生化特性,GmMAN19-1是一种来自大豆(Glycine max)的GH5_7 β-甘露聚糖酶,这种作物具有重要的农业意义。GmMAN19-1在萌发后的子叶中特异性表达,在pH 4.6和40°C时表现出最佳的嗜酸活性。在载子形式和与甘露糖戊糖(M5)配合物下,分别测定了GmMAN19-1在1.39和2.62 Å时的晶体结构。结构采用典型的(α/β)8 TIM桶状褶皱,活性位点槽呈v型。值得注意的是,鉴定出两种不同的M5结合模式,表明涉及水解和转糖基化活性的双重功能。位点定向诱变进一步验证了关键的催化和底物相互作用残基:E186A消除了酶活性,而Q267W改变了转糖基化产物谱,增强了对分支底物的活性。结合槽可以容纳半乳糖侧链,支持对半乳糖甘露聚糖的部分活性。这些发现为植物β-甘露聚糖酶的底物特异性和催化机制提供了全面的见解,并确立了GmMAN19-1在食品加工、生物质转化和工业生物技术方面的潜在应用,特别是由于其嗜酸性质和对分支甘露聚糖的增强活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


