P2RX7 modulates the function of human microglia-like cells and mediates the association of IL18 with Alzheimer's disease traits
IF 5.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
The immune system plays a dynamic role in neurodegenerative diseases, and purinergic receptors allow immune cells to recognize neuronal signaling, cell injury, or stress. Purinergic Receptor 7 (P2RX7) can modulate inflammatory cascades, and its expression is upregulated in Alzheimer's disease (AD) brain tissue. P2RX7 expression is enriched in microglia, and elevated levels are found in microglia surrounding amyloid-beta (Aβ) plaques in the brain. Despite evidence linking P2RX7 to AD, the mechanisms by which it shapes microglial responses and contributes to AD remain poorly defined. Here, we utilize a human monocyte-derived microglia-like cell model (MDMi) to interrogate P2RX7 activation and downstream consequences on microglial function. Specifically, we measured IL1β and IL18 production and Aβ1–42 uptake following ATP-induced P2RX7 activation. Our results show that ATP-stimulation of MDMi triggers upregulation of IL1β and IL18 expression, which is blocked with the A740003 P2RX7 antagonist. Elevated extracellular ATP also impaired Aβ1–42 uptake, an effect reversed by P2RX7 inhibition with A740003. In addition, pretreatment of MDMi with IL-1Ra limited ATP-driven IL1β and IL18 gene expression upregulation, indicating that ATP immunomodulation of P2RX7 is IL-1R dependent. Critically, analysis of postmortem human brain revealed that P2RX7 expression significantly mediated the association between IL18, but not IL1β, and multiple AD traits, including amyloid load, tau tangle density, global AD pathology burden, cortical and neocortical Lewy bodies, cognitive decline, and clinical dementia. Consistent with these findings, in our MDMi model, P2RX7 gene expression correlated with IL18 but not IL1β. Together, these findings highlight P2RX7 as a critical integrator of extracellular danger signals and microglial immune function, underscoring its potential as a therapeutic target in neurodegeneration.
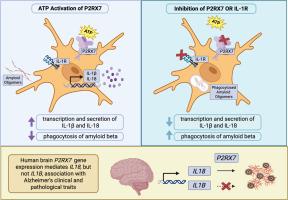
P2RX7调节人小胶质样细胞的功能,并介导il - 18与阿尔茨海默病特征的关联。
免疫系统在神经退行性疾病中起着动态作用,嘌呤能受体允许免疫细胞识别神经元信号、细胞损伤或应激。嘌呤能受体7 (P2RX7)可以调节炎症级联反应,其在阿尔茨海默病(AD)脑组织中的表达上调。P2RX7在小胶质细胞中表达丰富,在大脑淀粉样蛋白斑块周围的小胶质细胞中表达水平升高。尽管有证据表明P2RX7与阿尔茨海默病有关,但它形成小胶质细胞反应并导致阿尔茨海默病的机制仍不清楚。在这里,我们利用人类单核细胞衍生的小胶质样细胞模型(MDMi)来询问P2RX7的激活及其对小胶质功能的下游影响。具体来说,我们测量了atp诱导的P2RX7激活后il - 1β和il - 18的产生和a - β1-42的摄取。我们的研究结果表明,atp刺激MDMi可触发il - 1β和il - 18表达上调,而这一上调被A740003 P2RX7拮抗剂阻断。细胞外ATP升高也会损害a - β1-42的摄取,而A740003抑制P2RX7可逆转这一效应。此外,IL-1Ra预处理MDMi限制了ATP驱动的il -1 β和il - 18基因表达上调,表明P2RX7的ATP免疫调节依赖于IL-1R。重要的是,对人类死后大脑的分析显示,P2RX7的表达显著介导了il - 18(而非il - 1β)与阿尔茨海默病多种特征之间的关联,包括淀粉样蛋白负荷、tau蛋白结密度、阿尔茨海默病整体病理负担、皮层和新皮层路易体、认知能力下降和临床痴呆。与这些发现一致,在我们的MDMi模型中,P2RX7基因表达与il - 18相关,而与il - 1β无关。总之,这些发现强调了P2RX7作为细胞外危险信号和小胶质免疫功能的关键整合者,强调了其作为神经退行性疾病治疗靶点的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neurobiology of Disease
医学-神经科学
CiteScore
11.20
自引率
3.30%
发文量
270
审稿时长
76 days
期刊介绍:
Neurobiology of Disease is a major international journal at the interface between basic and clinical neuroscience. The journal provides a forum for the publication of top quality research papers on: molecular and cellular definitions of disease mechanisms, the neural systems and underpinning behavioral disorders, the genetics of inherited neurological and psychiatric diseases, nervous system aging, and findings relevant to the development of new therapies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


