Dual-Mode Wheat Germ Agglutinin Labeling – A Versatile Cell Segmentation Strategy for High-Resolution LA-ICP-TOFMS Bioimaging
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
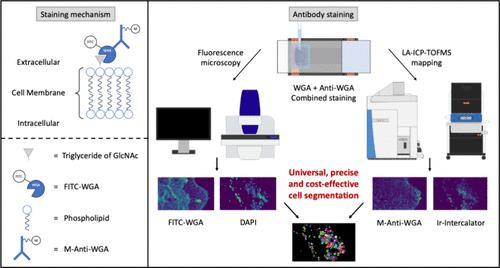
Single-cell analysis by laser ablation inductively coupled plasma time-of-flight mass spectrometry (LA-ICP-TOFMS) enables high-resolution mapping of elemental distributions and cellular phenotypes. Segmentation of individual cells necessitates labeling of both nuclei and membranes, the latter often requiring extensive tissue-specific optimization. In this study, we present a broadly applicable segmentation protocol based on wheat germ agglutinin (WGA), a lectin that binds to N-acetylglucosamine and sialic acid residues ubiquitously expressed on the cell membrane. By combining fluorescently labeled WGA with a metal-tagged anti-WGA antibody, we introduce a dual-labeling strategy compatible with both fluorescence microscopy and LA-ICP-TOFMS, enabling cross-validation of membrane labeling and enhancing segmentation accuracy. With recent advancements in laser ablation technology, such as higher repetition rates and submicrometer spot sizes, high-resolution imaging across large sample areas has become increasingly feasible. The robust, high-contrast membrane labeling achieved with our method facilitates precise cell segmentation at these resolutions and enhances the quality of the downstream single-cell data analysis. Beyond that, our approach reduces staining costs, streamlines workflows, and provides a scalable alternative to existing membrane-labeling strategies.
双模式小麦胚芽凝集素标记-高分辨率LA-ICP-TOFMS生物成像的通用细胞分割策略
单细胞分析通过激光烧蚀电感耦合等离子体飞行时间质谱(LA-ICP-TOFMS)实现元素分布和细胞表型的高分辨率映射。单个细胞的分割需要细胞核和膜的标记,后者通常需要广泛的组织特异性优化。在这项研究中,我们提出了一种基于小麦胚芽凝集素(WGA)的广泛适用的分割方案,WGA是一种结合在细胞膜上普遍表达的n -乙酰氨基葡萄糖和唾液酸残基的凝集素。通过将荧光标记的WGA与金属标记的抗WGA抗体相结合,我们引入了一种与荧光显微镜和LA-ICP-TOFMS兼容的双标记策略,实现了膜标记的交叉验证,提高了分割精度。随着激光烧蚀技术的进步,如更高的重复率和亚微米光斑尺寸,在大样本区域进行高分辨率成像变得越来越可行。用我们的方法实现的鲁棒性,高对比度的膜标记有助于在这些分辨率下精确的细胞分割,并提高下游单细胞数据分析的质量。除此之外,我们的方法降低了染色成本,简化了工作流程,并为现有的膜标记策略提供了可扩展的替代方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


