Paeoniflorin attenuates hyperprolactinemia by targeting the pituitary mPRα-mediated, dopamine receptor-independent signaling in vivo and in vitro
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Although dopamine receptor agonists are the primary therapies for prolactinoma and hyperprolactinemia, a population of patients are resistant or intolerant. Our previous findings have suggested that paeoniflorin, a major active ingredient contained in some frequently-used traditional Chinese formulas for hyperprolactinemia-related disorders, inhibits prolactin secretion via both dopamine receptor-dependent and independent pathways. Here, it was found that paeoniflorin ameliorated the antipsychotic olanzapine-induced hyperprolactinemia in rats and suppressed prolactin protein expression in GH3 rat pituitary tumor cells lacking dopamine receptor. Further, paeoniflorin suppressed olanzapine-induced overexpression of prolactin and estrogen receptor (ER) α proteins, but it reversed the downregulated protein expression of membrane progesterone receptor (mPR) α both in the pituitary of the rats and in GH3 cells. Network pharmacology analysis predicted that mPRα served as a core therapeutic target of paeoniflorin in hyperprolactinemia, with the transforming growth factor (TGF) β signaling pathway potentially mediating this pharmacological interaction. Molecular docking predicted that paeoniflorin interacted with mPRα. Silencing mPRα abolished paeoniflorin-mediated suppression of prolactin expression, confirming its essential role in mediating the effects of paeoniflorin. In addition, paeoniflorin upregulated TGFβ1 and downregulated ERα in an mPRα-dependent manner. Notably, ERα overexpression reversed inhibition of paeoniflorin on prolactin, while TGFβ1 inhibition attenuated the effect of paeoniflorin on ERα, highlighting the critical contributions of TGFβ1 and ERα to this regulatory pathway. Thus, our results in vivo and in vitro suggest that paeoniflorin attenuates hyperprolactinemia by targeting the pituitary mPRα-mediated, dopamine receptor-independent signaling, in which, TGFβ1 and ERα participate.
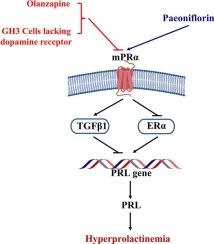
在体内和体外实验中,芍药苷通过靶向垂体mpr α介导的、不依赖多巴胺受体的信号通路来减轻高泌乳素血症。
尽管多巴胺受体激动剂是治疗催乳素瘤和高催乳素血症的主要药物,但仍有一部分患者存在耐药性或不耐受。我们之前的研究结果表明,在一些治疗高催乳素血症相关疾病的常用中药方剂中,芍药苷是一种主要的活性成分,它可以通过多巴胺受体依赖和独立途径抑制催乳素的分泌。本研究发现,芍药苷可改善抗精神病药奥氮平诱导的大鼠高催乳素血症,抑制缺乏多巴胺受体的GH3大鼠垂体肿瘤细胞中催乳素蛋白的表达。芍药苷抑制了奥氮平诱导的大鼠垂体和GH3细胞中泌乳素和雌激素受体(ER) α蛋白的过表达,逆转了膜孕酮受体(mPR) α蛋白的下调表达。网络药理学分析预测mPRα是芍药苷治疗高泌乳素血症的核心靶点,而转化生长因子(TGF) β信号通路可能介导了这种药物相互作用。分子对接预测芍药苷与mPRα相互作用。沉默mPRα可消除芍药苷介导的催乳素表达抑制,证实其在介导芍药苷作用中的重要作用。此外,芍药苷以mpr α依赖的方式上调tgf - β1,下调ERα。值得注意的是,ERα过表达逆转了芍药苷对催乳素的抑制作用,而TGFβ1的抑制则减弱了芍药苷对ERα的抑制作用,这突出了TGFβ1和ERα在这一调控途径中的重要作用。因此,我们在体内和体外的研究结果表明,芍药苷通过靶向垂体mpr α介导的、不依赖多巴胺受体的信号通路来减轻高泌乳素血症,TGFβ1和ERα参与了这一信号通路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


