Self-powered energy-efficient ammonia electrosynthesis via zinc-nitrite battery with bifunctional Pd-Co(OH)2 electrocatalyst
IF 9.7
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The electrochemical nitrite reduction reaction (NO2RR) has emerged as a promising strategy for sustainable ammonia (NH3) synthesis. However, conventional NO2RR systems suffer from high energy consumption and low efficiency due to the substantial kinetic overpotential. To address this challenge, we develop an innovative approach by coupling Pd with Co(OH)2 to effectively regulate the active hydrogen (∗H) dynamics for NO2−-to-NH3 conversion at low potentials. Benefiting from the thermodynamically favorable hydrogen spillover in the Pd-Co(OH)2/CF, an impressively high Faradaic efficiency (∼96.36 %) and NH3 production rate (∼44.69 mg h−1 cm−2) are realized at −0.2 V vs RHE. Inspired by the outstanding activity of Pd-Co(OH)2/CF for anodic formaldehyde oxidation reaction (FOR), the designed FOR-NO2RR electrolysis system demonstrates remarkable energy efficiency (10.75 kWh kg−1 NH3@100 mA cm−2), significantly outperforming conventional OER-NO2RR system (23.1 kWh kg−1 NH3). Furthermore, a Zn-NO2- battery powered FOR-NO2RR electrolysis system is proposed to enable NH4Cl fertilizer production and formaldehyde degradation. This work establishes a new paradigm for sustainable electrochemical systems that simultaneously address environmental remediation and value-added chemical production at unprecedented energy efficiency.
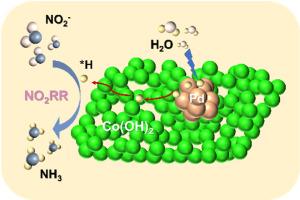
双功能Pd-Co(OH)2电催化剂在锌-亚硝酸盐电池上的自供电高效氨电合成
电化学亚硝酸盐还原反应(NO2RR)已成为可持续合成氨(NH3)的一种有前途的策略。然而,由于大量的动力学过电位,传统的NO2RR系统存在高能耗和低效率的问题。为了解决这一挑战,我们开发了一种创新的方法,通过将Pd与Co(OH)2偶联来有效调节低电位下NO2−到nh3转化的活性氢(∗H)动力学。得益于Pd-Co(OH)2/CF中有利的热力学氢溢出,在−0.2 V vs RHE下实现了令人印象深刻的法拉第效率(~ 96.36%)和NH3产率(~ 44.69 mg h−1 cm−2)。受Pd-Co(OH)2/CF在阳极甲醛氧化反应(for)中的杰出活性的启发,设计的for - no2rr电解系统具有显著的能源效率(10.75 kWh kg−1 NH3@100 mA cm−2),显著优于传统的OER-NO2RR系统(23.1 kWh kg−1 NH3)。此外,提出了一种由Zn-NO2电池供电的FOR-NO2RR电解系统,以实现NH4Cl肥料的生产和甲醛的降解。这项工作为可持续电化学系统建立了一个新的范例,同时以前所未有的能源效率解决环境修复和增值化学品生产问题。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Physics
Materials Science-General Materials Science
CiteScore
14.00
自引率
7.80%
发文量
284
审稿时长
15 days
期刊介绍:
Materials Today Physics is a multi-disciplinary journal focused on the physics of materials, encompassing both the physical properties and materials synthesis. Operating at the interface of physics and materials science, this journal covers one of the largest and most dynamic fields within physical science. The forefront research in materials physics is driving advancements in new materials, uncovering new physics, and fostering novel applications at an unprecedented pace.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


