Mutation of ube3a causes developmental abnormalities and autism-like molecular and behavioral alterations in zebrafish
IF 3.7
3区 医学
Q2 NEUROSCIENCES
引用次数: 0
Abstract
Mutations in the UBE3A gene are responsible for neurodevelopmental disorders (NDDs), including Angelman syndrome (AS), which is characterized by developmental delays, impaired motor coordination, and cognitive disabilities. In recent years, UBE3A mutations have also been linked to autism spectrum disorders (ASD), due to their significant role in synaptic plasticity and cognitive function. Although substantial research has utilized mammalian models, the zebrafish (Danio rerio) provides unique opportunities to investigate gene functions owing to their transparent embryos, rapid development, and suitability for large-scale genetic and behavioral studies. In this study, we characterized a zebrafish model harboring a point mutation (T > A) in exon 3 of the zebrafish ube3a gene, which induces a stop codon resulting in a truncated protein. We performed comprehensive developmental, behavioral, and molecular analyses to investigate the impact of Ube3a dysfunction at both larval and adult stages. We observed alterations in embryonic development, significant locomotor deficits, including stereotypic movements, and reduced social preference and aggressiveness. Furthermore, RNA sequencing analysis of both larvae and adults revealed dysregulation in chromatin, nucleosome, protein-DNA, and primary cilia-related genes. Our findings provide a functional characterization of the ube3a mutation in zebrafish at both larval and adult stages. This zebrafish model offers new insights into the roles of UBE3A in neurodevelopment and behavior, expanding our understanding of its dysfunction in NDDs.
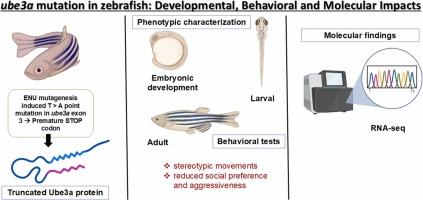
ube3a突变导致斑马鱼发育异常和自闭症样的分子和行为改变。
UBE3A基因突变是神经发育障碍(ndd)的原因,包括Angelman综合征(AS),其特征是发育迟缓,运动协调受损和认知障碍。近年来,由于UBE3A突变在突触可塑性和认知功能中发挥重要作用,也被认为与自闭症谱系障碍(ASD)有关。尽管大量研究利用了哺乳动物模型,但斑马鱼(Danio rerio)由于其透明的胚胎,快速发育以及适合大规模遗传和行为研究,为研究基因功能提供了独特的机会。在这项研究中,我们描述了斑马鱼ube3a基因外显子3中含有点突变(T > a)的模型,该突变诱导一个停止密码子导致一个截断的蛋白质。我们进行了全面的发育、行为和分子分析,以调查Ube3a功能障碍在幼虫和成虫阶段的影响。我们观察到胚胎发育的改变,显著的运动缺陷,包括刻板运动,以及社会偏好和攻击性的降低。此外,幼虫和成虫的RNA测序分析显示染色质、核小体、蛋白质- dna和初级纤毛相关基因失调。我们的研究结果提供了斑马鱼幼虫期和成虫期ube3a突变的功能特征。该斑马鱼模型为UBE3A在神经发育和行为中的作用提供了新的见解,扩大了我们对其在ndd中的功能障碍的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research Bulletin
医学-神经科学
CiteScore
6.90
自引率
2.60%
发文量
253
审稿时长
67 days
期刊介绍:
The Brain Research Bulletin (BRB) aims to publish novel work that advances our knowledge of molecular and cellular mechanisms that underlie neural network properties associated with behavior, cognition and other brain functions during neurodevelopment and in the adult. Although clinical research is out of the Journal''s scope, the BRB also aims to publish translation research that provides insight into biological mechanisms and processes associated with neurodegeneration mechanisms, neurological diseases and neuropsychiatric disorders. The Journal is especially interested in research using novel methodologies, such as optogenetics, multielectrode array recordings and life imaging in wild-type and genetically-modified animal models, with the goal to advance our understanding of how neurons, glia and networks function in vivo.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


