Theta-shaking mitigates cognitive-emotional decline via subiculum and ventral septum metabolic plasticity
引用次数: 0
Abstract
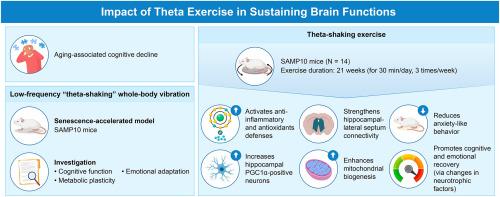
Aging-associated cognitive decline remains a major challenge in gerontology; few non-invasive interventions provide both mechanistic insight and translational feasibility. We investigated whether low-frequency “theta-shaking” whole-body vibration (5 Hz) could modulate cognitive function, emotional behavior, and metabolic plasticity in a senescence-accelerated mouse model. Senescence-accelerated mouse prone-10 mice were exposed to theta-shaking stimulation for 30 weeks. Spatial memory was assessed using Y-maze spontaneous alternation test, and anxiety-related behavior was evaluated using marble burying test. Histological and immunohistochemical analyses were conducted to assess neuronal density and protein expression in specific brain regions. Theta-shaking subjected mice exhibited delayed yet significant improvements in spatial memory at 20 (p = 0.017) and 30 (p = 0.018) weeks. Anxiety-related behavior shows a biphasic pattern: an initial increase at 20 weeks (p < 0.001) followed by stabilization at 30 weeks. Histological analysis revealed preserved neuronal density in the subiculum (p < 0.001) and elevated proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) expression in the Cornu Ammonis 1, subiculum, and lateral septum (all p < 0.05). Notably, mitochondrial biogenesis appeared to be intervention's primary target, as shown by robust PGC1α upregulation, while brain-derived neurotrophic factor revealed a trend-level increase (p = 0.062), and neurotrophin-3 expression remained unchanged. Frequency-tuned mechanical stimulation induced region-specific neural neurometabolic adaptations, supporting theta-shaking as a non-pharmacological, low-exertion strategy to counteract brain aging. These findings offer promising translational potential, especially for individuals with limited mobility.
脑波震动通过枕下和腹隔代谢可塑性减轻认知情绪下降
与衰老相关的认知能力下降仍然是老年学的主要挑战;很少有非侵入性干预既能提供机理见解又能提供转化可行性。在衰老加速小鼠模型中,我们研究了低频“抖theta”全身振动(5hz)是否可以调节认知功能、情绪行为和代谢可塑性。衰老加速小鼠倾向-10小鼠暴露于摇脑波刺激30周。采用y迷宫自发交替测验评估空间记忆,采用弹珠掩埋测验评估焦虑相关行为。通过组织学和免疫组织化学分析来评估特定脑区域的神经元密度和蛋白质表达。在第20周(p = 0.017)和第30周(p = 0.018)时,震荡实验小鼠在空间记忆方面表现出延迟但显著的改善。焦虑相关行为表现出双相模式:在20周时开始增加(p < 0.001),随后在30周时稳定下来。组织学分析显示,枕骨下的神经元密度保持不变(p < 0.001),鹦鹉角1、枕骨下和外侧隔的增殖因子激活受体γ辅助激活因子1- α (PGC1α)表达升高(p < 0.05)。值得注意的是,线粒体生物发生似乎是干预的主要目标,如PGC1α的强劲上调,而脑源性神经营养因子呈趋势水平升高(p = 0.062),神经营养因子-3的表达保持不变。频率调谐的机械刺激诱导了特定区域的神经代谢适应,支持theta-shaking作为一种非药物、低消耗的策略来对抗大脑衰老。这些发现提供了有希望的转化潜力,特别是对行动不便的个体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


