Allosteric pockets in the measles and Nipah virus polymerases: Mechanobiological insights and AI-driven drug discovery opportunities
引用次数: 0
Abstract
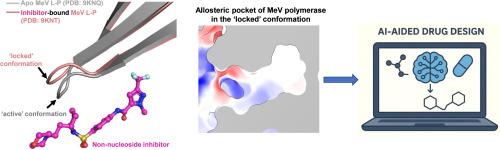
Nonsegmented negative-sense RNA viruses (nsNSVs)—including highly pathogenic pathogens such as measles virus (MeV), Nipah virus (NiV), Hendra virus (HeV), Ebola virus (EBOV), and others—pose major global health threats, yet most lack approved antiviral therapeutics. In the recent study, high-resolution cryo-electron microscopy (cryo-EM) revealed previously unrecognized allosteric pockets in the large (L) polymerase proteins of MeV and NiV, spatially distinct from the catalytic nucleotide-binding site. We further demonstrated that the non-nucleoside inhibitor ERDRP-0519 engages these pockets to allosterically ‘lock’ the polymerase in a mechanically inactive state. These findings reveal an allosteric mechanism of inhibition rooted in the conformational mechanics of the enzyme and highlight opportunities for integrating artificial intelligence (AI)-aided drug discovery (AIDD) into rational drug design.
麻疹和尼帕病毒聚合酶的变构口袋:机械生物学见解和人工智能驱动的药物发现机会
非分段负义RNA病毒(nsNSVs)——包括麻疹病毒(MeV)、尼帕病毒(NiV)、亨德拉病毒(HeV)、埃博拉病毒(EBOV)等高致病性病原体——对全球健康构成重大威胁,但大多数病毒缺乏经批准的抗病毒治疗方法。在最近的研究中,高分辨率冷冻电镜(cryo-EM)发现MeV和NiV的大(L)聚合酶蛋白中先前未被识别的变弹性口袋,在空间上与催化核苷酸结合位点不同。我们进一步证明,非核苷类抑制剂ERDRP-0519与这些小口袋结合,以变弹性“锁定”聚合酶,使其处于机械失活状态。这些发现揭示了基于酶的构象机制的抑制变构机制,并突出了将人工智能(AI)辅助药物发现(AIDD)整合到合理药物设计中的机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


