Exploration of zileuton protective mechanisms against vancomycin-associated nephrotoxicity
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Vancomycin is one of the most commonly used parenteral antibiotics for treating drug-resistant bacterial infections, however, it is hindered by nephrotoxicity. We previously demonstrated that zileuton could delay the onset of vancomycin-associated nephrotoxicity in rats. Here, we sought to understand the mechanism(s) of zileuton renal protection. Sprague-Dawley rats were administered vancomycin (200 mg/kg) and zileuton (1 and 4 mg/kg) daily for 10 days. After 3 days, kidneys were collected from select animals for histopathological analysis of renal injury. Single-dose vancomycin serum pharmacokinetics and renal tissue spatial distribution with adjuvant zileuton were evaluated. In vitro, proximal tubular cells were exposed to vancomycin and zileuton; cell viability, vancomycin accumulation, ROS levels, and p62/KEAP1 and ferroptosis-related protein levels were measured. Vancomycin was associated with increased serum creatinine and proximal tubule injury in rats including tubular cell necrosis, cytoplasmic vacuolization, interstitial edema, and mononuclear inflammatory cell infiltration. Adjuvant zileuton reduced renal injury and serum creatinine elevation without altering vancomycin serum pharmacokinetics or renal tissue distribution. In vitro, vancomycin exposure resulted in cellular injury, increased ROS, and significantly decreased HO-1 levels. Concomitant zileuton reduced cellular injury, decreased ROS, and rescued HO-1 levels. These preliminary findings indicate that zileuton may be protective against vancomycin-associated renal injury potentially by rescuing HO-1 levels and reducing oxidative stress in proximal tubular cells. Attenuation of nephrotoxicity would allow the optimal clinical use of vancomycin to treat drug-resistant bacterial infections, which could reduce patient harm and hospitalization costs.
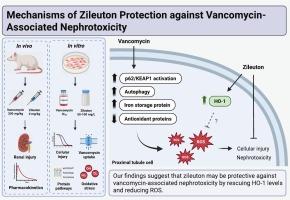
紫丁香对万古霉素相关肾毒性的保护机制探讨。
万古霉素是治疗耐药细菌感染最常用的肠外抗生素之一,然而,它受到肾毒性的阻碍。我们之前证明zileuton可以延缓万古霉素相关大鼠肾毒性的发生。在这里,我们试图了解子午线对肾脏保护的机制。Sprague-Dawley大鼠每日给药万古霉素(200 mg/kg)和zileuton(1和4 mg/kg),连续10 d。3 d后,取肾进行肾损伤组织病理学分析。观察单剂量万古霉素的血清药代动力学和佐剂紫金酮的肾组织空间分布。在体外,将近端小管细胞暴露于万古霉素和zileuton;测定细胞活力、万古霉素积累、ROS水平、p62/KEAP1和凋亡相关蛋白水平。万古霉素与大鼠血清肌酐升高和近端小管损伤相关,包括小管细胞坏死、细胞质空泡化、间质水肿和单核炎症细胞浸润。佐剂zileuton减轻了肾损伤和血清肌酐升高,而没有改变万古霉素的血清药代动力学和肾组织分布。在体外,万古霉素暴露导致细胞损伤,ROS升高,HO-1水平显著降低。伴随的zileuton减轻了细胞损伤,降低了ROS,并挽救了HO-1水平。这些初步发现表明zileuton可能通过挽救HO-1水平和减少近端小管细胞的氧化应激来保护万古霉素相关的肾损伤。肾毒性的减弱将使万古霉素在临床上用于治疗耐药细菌感染成为可能,从而减少对患者的伤害和住院费用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


