Specific parvalbumin-positive optogenetic stimulations in specific brain regions restore navigational flexibility in an acute MK801 mouse model of schizophrenia
IF 2.8
3区 医学
Q2 NEUROSCIENCES
引用次数: 0
Abstract
Impairments in decision-making and behavioral flexibility in patients with schizophrenia (SCZ) are currently among the most investigated aspects of SCZ. Increased GLUergic excitatory activity and decreased GABAergic inhibitory activity induce mPFC-vHPC γ/θ band desynchronization in many tasks where behavioral flexibility is tested. However, these tasks used “perceptual” decision-making/flexibility but not navigational decision-making/flexibility. Our study investigated the role of frequency-specific optogenetic stimulation of GABAergic parvalbumin-positive (PV+) interneurons in two pivotal brain structures used in flexibility (mPFC) and navigation (vHPC), at frequencies resembling the γ/θ band (50 Hz, γ-like; and 10 Hz, θ-like) in an acute MK801 mouse model of navigational inflexibility. We used a modified version of the active place avoidance task on a rotating arena. The behavioral results revealed that frequency-specific optogenetic stimulation of the mPFC or vHPC had different effects on restoring navigational flexibility. Moreover, immunohistochemical assays confirmed that optogenetic stimulations activated PV+ interneurons that were transfected with the optogenetic actuators, advancing our understanding of the pivotal role of PV+ activity in SCZ-like navigational decision-making/flexibility.
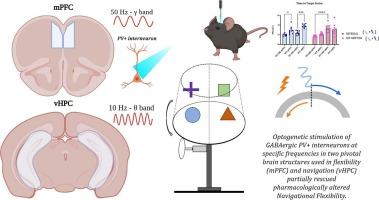
在一个急性MK801精神分裂症小鼠模型中,特定脑区域的特异性小蛋白阳性光遗传刺激恢复了导航灵活性
精神分裂症患者的决策和行为灵活性障碍是目前研究最多的精神分裂症方面。在许多测试行为灵活性的任务中,增加的GLUergic兴奋活性和降低的GABAergic抑制活性诱导mPFC-vHPC γ/θ波段不同步。然而,这些任务使用“感知”决策/灵活性,而不是导航决策/灵活性。我们的研究调查了频率特异性光遗传刺激GABAergic parvalbumin阳性(PV+)中间神经元在两个用于灵活性(mPFC)和导航(vHPC)的关键脑结构中的作用,频率类似于γ/θ波段(50 Hz, γ-样;和10 Hz, θ-样)在急性MK801小鼠导航不灵活性模型中。我们在一个旋转的舞台上使用了一个改进版本的主动位置回避任务。行为学结果显示,频率特异性光遗传刺激mPFC或vHPC对恢复导航灵活性有不同的影响。此外,免疫组织化学分析证实,光遗传刺激激活了转染了光遗传致动器的PV+中间神经元,进一步加深了我们对PV+活性在scz样导航决策/灵活性中的关键作用的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuroscience
医学-神经科学
CiteScore
6.20
自引率
0.00%
发文量
394
审稿时长
52 days
期刊介绍:
Neuroscience publishes papers describing the results of original research on any aspect of the scientific study of the nervous system. Any paper, however short, will be considered for publication provided that it reports significant, new and carefully confirmed findings with full experimental details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


