NFE2L2 protects against Sorafenib-induced Ferroptosis and cardiotoxicity by activating the HO1/ferritin pathway
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Sorafenib, a tyrosine kinase inhibitor, has demonstrated efficacy in the treatment of hepatocellular carcinoma and clear cell renal carcinoma. However, its clinical application is limited by cardiotoxicity. Here, we show that NFE2L2, a transcription factor that regulates oxidative stress and iron homeostasis, mitigates Sorafenib-induced cardiotoxicity. Sorafenib increases NFE2L2 expression in cardiomyocytes, while ferroptosis inhibitors such as ferrostatin-1 (Fer-1) and deferoxamine (DFO) attenuate this effect, indicating that ferroptosis is involved in NFE2L2 activation. Further studies revealed that NFE2L2 knockdown exacerbates Sorafenib-induced cardiomyocyte ferroptosis, which is characterized by increased lipid peroxidation and reactive oxygen species (ROS) production. Conversely, NFE2L2 agonist sulforaphane (SFN) mitigated Sorafenib-induced ferroptosis. Mechanistically, NFE2L2 activates the heme oxygenase-1 (HO-1)/ferritin axis, which alleviates oxidative stress and promotes iron homeostasis in cardiomyocytes, thereby mitigating Sorafenib-induced ferroptosis. Interestingly, Sorafenib activates NFE2L2 via the endoplasmic reticulum (ER) stress-related kinase EIF2AK3, rather than the SQSTM1-KEAP1 pathway. This finding reveals a novel role for ER stress-dependent pathways in counteracting Sorafenib-induced cardiotoxicity. Finally, we found that SFN alleviates Sorafenib-induced cardiotoxicity in vivo, providing a new therapeutic strategy for managing drug-induced cardiac injury. Taken together, these data suggest that NFE2L2 and its downstream pathways, including the HO-1/ferritin axis, may represent promising therapeutic targets for mitigating Sorafenib-induced cardiotoxicity. Further investigation of NFE2L2 agonists could enhance the safety of Sorafenib therapy.
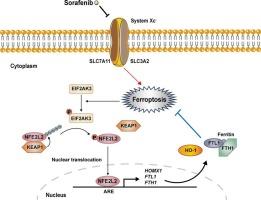
NFE2L2通过激活HO1/铁蛋白通路,对索拉非尼诱导的铁凋亡和心脏毒性具有保护作用
索拉非尼是一种酪氨酸激酶抑制剂,在治疗肝细胞癌和透明细胞肾癌方面已被证明有效。然而,其临床应用受到心脏毒性的限制。在这里,我们发现NFE2L2,一种调节氧化应激和铁稳态的转录因子,减轻了索拉非尼诱导的心脏毒性。索拉非尼增加了心肌细胞中NFE2L2的表达,而铁下垂抑制剂如铁他汀-1 (fer1)和去铁胺(DFO)减弱了这种作用,表明铁下垂参与了NFE2L2的激活。进一步的研究表明,NFE2L2敲低加剧了索拉非尼诱导的心肌细胞铁下沉,其特征是脂质过氧化和活性氧(ROS)的产生增加。相反,NFE2L2激动剂萝卜硫素(SFN)减轻了索拉非尼诱导的铁下垂。机制上,NFE2L2激活血红素加氧酶-1 (HO-1)/铁蛋白轴,减轻氧化应激,促进心肌细胞铁稳态,从而减轻索拉非尼诱导的铁凋亡。有趣的是,索拉非尼通过内质网(ER)应激相关激酶EIF2AK3激活NFE2L2,而不是通过SQSTM1-KEAP1途径。这一发现揭示了内质网应激依赖途径在对抗索拉非尼诱导的心脏毒性中的新作用。最后,我们发现SFN在体内可减轻索拉非尼诱导的心脏毒性,为治疗药物性心脏损伤提供了一种新的治疗策略。综上所述,这些数据表明NFE2L2及其下游通路,包括HO-1/铁蛋白轴,可能是缓解索拉非尼诱导的心脏毒性的有希望的治疗靶点。进一步研究NFE2L2激动剂可以提高索拉非尼治疗的安全性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


