Dual-Probe Strategy-Enabled Multiplex Photoelectrochemical Sensor for Highly Sensitive Screening of Diabetes and Related Complications
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Photoelectrochemical (PEC) biosensing has emerged as a vital tool in disease surveillance and therapeutic monitoring. However, most current PEC platforms are constrained to single biomarker detection, limiting their utility in comprehensive disease management. In this study, we report the development of a dual-target PEC biosensor by integrating silane molecules and β-cyclodextrin (β-CD) with carbon nitride materials, specifically designed for monitoring diabetes and its associated complication, uremia. The silane molecules enhance the photocurrent through an in situ sensitization effect triggered by α-glucosidase (α-Glu)-catalyzed hydrolysis products, while the complexation between β-CD and l-histidine (l-His) results in a contrasting photocurrent suppression due to steric hindrance. This dual-response mechanism enables precise and selective detection of both biomarkers. The developed biosensing platform displays remarkable detection capability for α-Glu, achieving a linear photocurrent response across the concentration range of 5–100 mU/mL, with the method demonstrating exceptional sensitivity down to 0.62 mU/mL. Simultaneously, l-His detection is achieved over a range of 0.01–1 mM, with a detection limit down to 7.2 μM. In addition to high sensitivity, the resultant sensing platform demonstrates excellent stability, reproducibility, and resistance to interference. By integrating in situ sensitization and host–guest complexation effects, this work presents a novel and robust strategy for multifunctional PEC biosensor design, offering new opportunities for efficient and comprehensive disease monitoring.
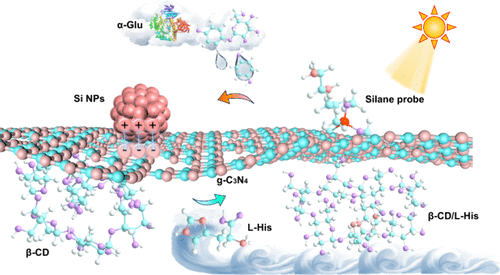
基于双探针策略的多路光电电化学传感器用于糖尿病及相关并发症的高灵敏度筛查
光电化学(PEC)生物传感已成为疾病监测和治疗监测的重要工具。然而,目前大多数PEC平台仅限于单一生物标志物检测,限制了它们在综合疾病管理中的应用。在这项研究中,我们将硅烷分子和β-环糊精(β-CD)与氮化碳材料结合,开发了一种双靶点PEC生物传感器,专门用于监测糖尿病及其相关并发症尿毒症。硅烷分子通过α-葡萄糖苷酶(α-Glu)催化的水解产物引发的原位敏化效应增强光电流,而β-CD与l-组氨酸(l-His)的络合作用由于位阻导致了对比鲜明的光电流抑制。这种双重反应机制能够精确和选择性地检测这两种生物标志物。所开发的生物传感平台对α-Glu具有显著的检测能力,在5-100 mU/mL的浓度范围内实现了线性光电流响应,灵敏度低至0.62 mU/mL。同时,l-His的检测范围为0.01-1 mM,检测限低至7.2 μM。除了高灵敏度外,由此产生的传感平台还具有出色的稳定性,可重复性和抗干扰性。通过整合原位致敏和主客体络合效应,本研究为多功能PEC生物传感器设计提供了一种新颖而强大的策略,为有效和全面的疾病监测提供了新的机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


