Comparative analysis of curcuminoid content, antioxidant capacity, and target-specific molecular docking of turmeric extracts sourced from Thailand
IF 4.7
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
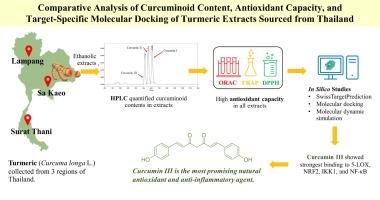
Curcuminoids are the active compounds richest in turmeric rhizomes (Curcuma longa L.), comprising curcumin I, demethoxycurcumin (curcumin II), and bisdemethoxycurcumin (curcumin III). This study hypothesized that particular curcumin derivatives could mitigate oxidative stress and inflammation response by targeting specific inflammatory mediators. Therefore, this study aimed to quantify the concentrations of these curcuminoid forms in local turmeric extracts from Thailand. Subsequently, the study analyzed their in vitro antioxidant properties, alongside molecular docking and dynamics simulations targeting key oxidative stress- and inflammation-related proteins. Samples were collected from three representative cultivated areas in Thailand: the eastern, southern, and northern regions. The ethanolic extracts from all samples exhibited relatively high total curcuminoid content (eastern: 15.1 %, southern: 25.9 %, and northern: 31.6 % w/w in extract), as determined by high-performance liquid chromatography. Curcumin I emerged as the predominant variant, followed closely by curcumin II and III. The ethanolic extracts from the three cultural areas demonstrated significant antioxidant activity, as assessed by ORAC, FRAP, and DPPH assays. Among the three curcuminoids, curcumin III exhibited the strongest predicted binding affinities in molecular docking studies toward antioxidant and anti-inflammatory targets, including 5-LOX, NRF2, IKK1, NF-κB, and NOX4. Molecular dynamics simulations corroborated these findings, revealing that curcumin III formed the most stable complexes, particularly with IKK1, as indicated by low RMSD values (2–3 Å), and high hydrogen bond occupancy. Thus, curcumin III exhibits potential in silico inhibition of inflammatory mediators, supporting its promise as a natural compound for antioxidant and anti-inflammatory nutraceutical development.
泰国姜黄提取物的类姜黄素含量、抗氧化能力和靶向分子对接的比较分析
姜黄素是姜黄根茎(Curcuma longa L.)中最丰富的活性化合物,包括姜黄素I、去甲氧基姜黄素(姜黄素II)和双去甲氧基姜黄素(姜黄素III)。本研究假设特定的姜黄素衍生物可以通过靶向特定的炎症介质来减轻氧化应激和炎症反应。因此,本研究旨在量化泰国当地姜黄提取物中这些姜黄素形式的浓度。随后,该研究分析了它们的体外抗氧化性能,以及针对关键氧化应激和炎症相关蛋白的分子对接和动力学模拟。样本采集自泰国三个具有代表性的种植区:东部、南部和北部地区。通过高效液相色谱法测定,所有样品的乙醇提取物均具有较高的姜黄素总含量(东部:15.1%,南部:25.9%,北部:31.6% w/w)。姜黄素I是主要的变体,其次是姜黄素II和III。通过ORAC、FRAP和DPPH检测,三个培养区的乙醇提取物显示出显著的抗氧化活性。在三种姜黄素中,姜黄素III在抗氧化和抗炎靶点(包括5-LOX、NRF2、IKK1、NF-κB和NOX4)的分子对接研究中表现出最强的预测结合亲和力。分子动力学模拟证实了这些发现,显示姜黄素III形成了最稳定的复合物,特别是与IKK1,正如低RMSD值(2-3 Å)和高氢键占用所表明的那样。因此,姜黄素III显示出对炎症介质的硅抑制潜力,支持其作为抗氧化和抗炎营养品开发的天然化合物的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry Molecular Sciences
Agricultural and Biological Sciences-Food Science
CiteScore
6.00
自引率
0.00%
发文量
83
审稿时长
82 days
期刊介绍:
Food Chemistry: Molecular Sciences is one of three companion journals to the highly respected Food Chemistry.
Food Chemistry: Molecular Sciences is an open access journal publishing research advancing the theory and practice of molecular sciences of foods.
The types of articles considered are original research articles, analytical methods, comprehensive reviews and commentaries.
Topics include:
Molecular sciences relating to major and minor components of food (nutrients and bioactives) and their physiological, sensory, flavour, and microbiological aspects; data must be sufficient to demonstrate relevance to foods and as consumed by humans
Changes in molecular composition or structure in foods occurring or induced during growth, distribution and processing (industrial or domestic) or as a result of human metabolism
Quality, safety, authenticity and traceability of foods and packaging materials
Valorisation of food waste arising from processing and exploitation of by-products
Molecular sciences of additives, contaminants including agro-chemicals, together with their metabolism, food fate and benefit: risk to human health
Novel analytical and computational (bioinformatics) methods related to foods as consumed, nutrients and bioactives, sensory, metabolic fate, and origins of foods. Articles must be concerned with new or novel methods or novel uses and must be applied to real-world samples to demonstrate robustness. Those dealing with significant improvements to existing methods or foods and commodities from different regions, and re-use of existing data will be considered, provided authors can establish sufficient originality.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


