Prediction of human pharmacokinetics of procymidone and its major phase I and II metabolites using a PBPK model with enterohepatic circulation
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
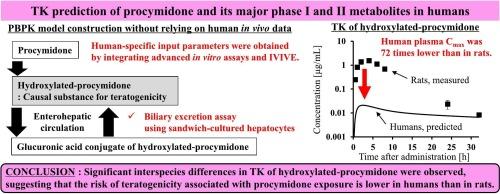
Physiologically based pharmacokinetic (PBPK) modeling is a valuable approach for addressing the scarcity of human toxicokinetic data, particularly in the risk assessment of agrochemicals. While numerous PBPK models have been developed to describe various pharmacokinetics processes, modeling the complex kinetics of enterohepatic circulation remains challenging. Procymidone, a widely used fungicide, undergoes initial metabolism to hydroxylated-procymidone, an active metabolite with antiandrogenic properties, followed by glucuronidation. In rabbits, monkeys, and chimeric mice with humanized liver, the glucuronic acid conjugate is rapidly excreted in urine. However, in rats, it is primarily excreted into bile, deconjugated in the gastrointestinal tract, and subsequently reabsorbed, leading to enterohepatic circulation. This species difference in biliary excretion is considered a key factor contributing to the elevated plasma levels of hydroxylated-procymidone in rats, which are associated with rat-specific developmental toxicity. To estimate human internal exposure to hydroxylated-procymidone, a PBPK model was developed incorporating quantitative prediction of complex pharmacokinetics. This model was constructed without relying on human in vivo data, instead integrating advanced in vitro systems, such as bile canalicular membrane transporter assays using sandwich-cultured human hepatocytes and in vitro-to-in vivo extrapolation. By comparing the predicted plasma concentration of hydroxylated-procymidone in humans with measured values in rats, significant interspecies differences were observed, suggesting that the risk of developmental toxicity associated with procymidone exposure is lower in humans than in rats.
利用肠肝循环PBPK模型预测人原胺酮及其主要I期和II期代谢物的药代动力学
基于生理的药代动力学(PBPK)建模是解决人类毒物动力学数据稀缺的一种有价值的方法,特别是在农用化学品的风险评估中。虽然已经开发了许多PBPK模型来描述各种药代动力学过程,但建立复杂的肠肝循环动力学模型仍然具有挑战性。原胺酮是一种广泛使用的杀菌剂,其初始代谢为羟基化原胺酮,是一种具有抗雄激素特性的活性代谢物,随后是葡萄糖醛酸化。在兔、猴和具有人源化肝脏的嵌合小鼠中,葡萄糖醛酸缀合物可迅速随尿液排出。然而,在大鼠中,它主要被排泄到胆汁中,在胃肠道中解结,随后再吸收,导致肠肝循环。这种胆道排泄的物种差异被认为是导致大鼠血浆中羟基化-原胺酮水平升高的关键因素,这与大鼠特异性发育毒性有关。为了估计人体内暴露于羟基化-原胺酮,建立了一个PBPK模型,结合复杂药代动力学的定量预测。该模型的构建不依赖于人体体内数据,而是整合了先进的体外系统,例如使用三明治培养的人肝细胞进行胆管膜转运蛋白检测和体外-体内外推。通过比较人类羟化-原酰米酮的预测血浆浓度与大鼠的测量值,观察到显着的种间差异,表明与暴露于原酰米酮相关的发育毒性风险在人类中低于在大鼠中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


