Development of a complex 3D in vitro alternative model to evaluate the safety of advanced materials
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Drug-induced liver injury (DILI) i.e., liver damage caused by pharmaceutical and other chemical substances, represents a significant clinical challenge in both its diagnosis and treatment, with DILI remaining a major factor in drug attrition and market withdrawal, accounting for approximately 50 % of reported cases of acute liver failure. For decades, conventional in vitro two-dimensional (2D) monolayer cultures and in vivo animal models have been the gold standard in preclinical studies of DILI. However, these models exhibit critical limitations in the accurate replication of metabolic functionality and complexity of human hepatic tissue, a particular disadvantage in the context of drug testing. Rooted in the 3Rs principle of Replacement, Reduction and Refinement, and driven by regulatory frameworks such as REACH (Regulation (EC) No 1907/2006) and Directive 2010/63/EU, which promote ethical alternatives in toxicological studies, one strategy for overcoming issues prevalent when assessing DILI has been the development and adoption of NAMs, or New Approach Methodologies. The use of these innovative and alternative advanced non-animal in vitro systems, which can include 3D cell spheroids, organoids and organ-on-chip (OoC) technology, have shown promising results in recapitulating tissue-mimetic and biologically relevant aspects of target tissue and organs, specifically regarding the liver. Supported by European Union (EU) initiatives like Horizon Europe, and the Joint Research Centres EU Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM), a core aim of NAMs is to bridge the pre-clinical knowledge gap when evaluating the safety of both drugs and advanced materials (AMs) by developing more predictive and mechanistic safety assessments. Challenges remain however regarding regulatory acceptance, model standardisation and integration into the existing risk assessment frameworks. In spite of these hurdles, NAMs have the potential to transform toxicology, by providing more human-relevant, efficient and ethical alternatives to conventional testing methods.
With this in mind, this work deals with the development of a state-of-the-art complex 3D hepatic spheroid model, cultured from both parenchymal (human hepatocellular carcinoma; HepG2) and non-parenchymal (a mixed population of Kupffer cells (KCs), hepatic stellate cells (HSCs) and liver sinusoidal endothelial (LSEC)) cells. This promising NAM not only reconstructs the intrinsic architecture of the human liver, but also facilitates chronic and repeated viability testing over 30 days. The models ability to assess both safety and efficacy to a panel of common hepatotoxins and two representative advanced materials was assessed by ATP quantification, with this study demonstrating the reliability of using NAMs-based systems for in vitro DILI assessment and their revolutionary potential for pre-clinical safety screening as a novel human-relevant liver model.
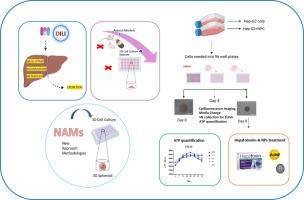
开发一个复杂的3D体外替代模型来评估先进材料的安全性。
药物性肝损伤(DILI),即由药物和其他化学物质引起的肝损伤,在诊断和治疗方面都是一个重大的临床挑战,DILI仍然是药物消耗和市场退出的主要因素,约占报告的急性肝衰竭病例的50% %。几十年来,传统的体外二维(2D)单层培养和体内动物模型一直是DILI临床前研究的金标准。然而,这些模型在准确复制人体肝脏组织的代谢功能和复杂性方面表现出严重的局限性,这在药物测试方面尤其不利。基于3Rs替代、减少和改进原则,并受到REACH(法规(EC) No 1907/2006)和指令2010/63/EU等监管框架的推动,这些监管框架促进了毒理学研究中的道德替代品,在评估DILI时克服普遍问题的一种策略是开发和采用NAMs或新方法方法。使用这些创新的先进非动物体外系统,包括3D细胞球体、类器官和器官芯片(OoC)技术,在重现目标组织和器官的模拟组织和生物学相关方面,特别是在肝脏方面,已经显示出有希望的结果。在Horizon Europe等欧盟倡议和欧盟动物试验替代方案联合研究中心(EURL ECVAM)的支持下,NAMs的核心目标是通过开发更具预测性和机械性的安全性评估,在评估药物和先进材料(AMs)安全性时弥合临床前知识差距。然而,在监管接受、模型标准化和融入现有风险评估框架方面,挑战仍然存在。尽管存在这些障碍,NAMs仍有潜力通过提供与人类更相关、更有效和更合乎道德的替代传统测试方法来改变毒理学。考虑到这一点,这项工作涉及最先进的复杂3D肝球体模型的发展,从实质(人肝细胞癌;HepG2)和非实质细胞(库普弗细胞(KCs)、肝星状细胞(hsc)和肝窦内皮细胞(LSEC)的混合群体)。这种有前途的NAM不仅重建了人类肝脏的内在结构,而且还促进了30 天内的慢性和重复活力测试。该模型对一组常见肝毒素和两种具有代表性的先进材料的安全性和有效性进行了ATP定量评估,该研究证明了使用基于nams的系统进行体外DILI评估的可靠性,以及它们作为一种新型人类相关肝脏模型在临床前安全性筛选方面的革命性潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


