Restoring resident tissue macrophages to combat aging and cancer
IF 19.4
Q1 CELL BIOLOGY
引用次数: 0
Abstract
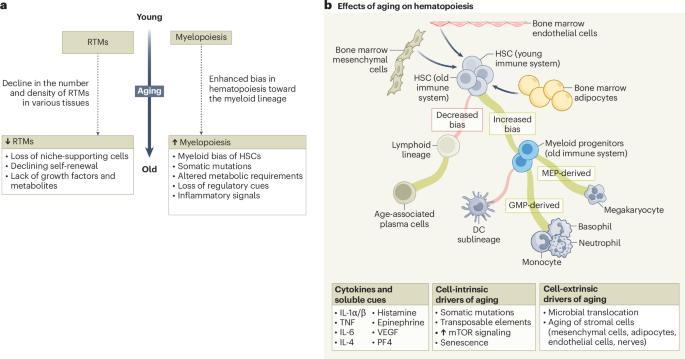
Perturbations to the immune system influence organismal aging, yet identifying effective therapeutic targets that mitigate aging-related tissue decline or the pathogenesis of aging-related diseases, such as cancer, remains challenging. In this Perspective, we focus on the dysfunction and loss of resident tissue macrophages (RTMs) with aging of certain tissues, which promote local inflammation, compromise tissue health and contribute to tumorigenesis. The abnormal genesis of RTMs from the bone marrow is a defining hallmark of both healthy and unhealthy aging. So, we propose that restoring RTMs—either by reshaping their niche and rescuing local self-renewal or by rejuvenating aging-associated myelopoiesis in the bone marrow—should be a major objective of interventions to promote healthy aging. We summarize the body of work supporting this conceptual framework and outline key future directions for the development of versatile myeloid-targeting therapies. Park and colleagues propose a conceptual framework in which dysregulation of resident tissue macrophages is both a central contributor to how an aging immune system causes diseases, including cancer, and a potential therapeutic target.
恢复常驻组织巨噬细胞对抗衰老和癌症。
对免疫系统的干扰会影响机体衰老,但确定有效的治疗靶点以减轻与衰老相关的组织衰退或衰老相关疾病(如癌症)的发病机制仍然具有挑战性。在这个角度上,我们关注的是随着某些组织的衰老,常驻组织巨噬细胞(RTMs)的功能障碍和损失,这促进了局部炎症,损害了组织健康并促进了肿瘤的发生。来自骨髓的RTMs的异常发生是健康和不健康衰老的决定性标志。因此,我们建议通过重塑其生态位和挽救局部自我更新或通过恢复骨髓中与衰老相关的骨髓生成来恢复rtm,应该是促进健康衰老的干预措施的主要目标。我们总结了支持这一概念框架的工作,并概述了未来发展多用途骨髓靶向治疗的关键方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


