Empagliflozin attenuates sorafenib-promoted renal ferroptosis and inflammation by targeting cyclooxygenase-2/prostaglandin E2 axis
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The increased risk of nephrotoxicity may impact the life quality and survival outcome in cancer patients receiving sorafenib therapy. Therefore, the development of novel strategy against sorafenib nephrotoxicity is an urgent work. Sodium-glucose co-transporter-2 (SGLT2) inhibitors such as empagliflozin have been approved for renal failure treatment. So far, the potential of empagliflozin against sorafenib nephrotoxicity has not yet been reported. The SGLT2 and apoptotic marker expressions in the sorafenib-treated renal proximal tubular cells (HK-2 cells) was investigated using immunoblot analysis. The cell viability was evaluated in HK-2 cells after sorafenib ± empagliflozin treatment using Alamar blue assay. The immunoblot analysis was applied to study the effect of sorafenib ± empagliflozin treatment on ferroptotic and proinflammatory stresses in HK-2 cells. The cell death, ferroptosis, lipid peroxidation, cytokine storm, and immune cells recruitments of kidneys was investigated in mice receiving a 28-day sorafenib ± empagliflozin administration using histopathological analyses. Sorafenib exposure dose-dependently upregulated SGLT2 in HK-2 cells, and empagliflozin significantly attenuated the sorafenib-induced cell death in HK-2 cells and mouse kidneys. Moreover, the sorafenib-stimulated iron deposition, oxidative DNA damage, lipid peroxidation, and glutathione peroxidase 4 (GPX4)/ SLC7A11 (xCT)-dependent ferroptosis were significantly alleviated by empagliflozin in mouse kidneys. The sorafenib-promoted cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) signaling as a ferroptosis driver was significantly blocked by empagliflozin in HK-2 cells and mouse kidneys. Empagliflozin also attenuated the sorafenib-stimulated-HMGB1/IL-1β proinflammatory signaling in vitro and in vivo. Furthermore, the sorafenib-promoted macrophage and neutrophil infiltrations were significantly reduced by empagliflozin in mouse kidneys. Collectively, empagliflozin may serve as a potent anti-ferroptotic and anti-inflammatory agent against sorafenib nephrotoxicity by targeting COX-2/PGE2 axis.
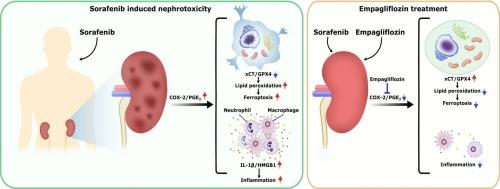
恩格列清通过靶向环氧化酶-2/前列腺素E2轴减轻索拉非尼促进的肾铁下垂和炎症。
肾毒性风险的增加可能会影响接受索拉非尼治疗的癌症患者的生活质量和生存结果。因此,开发抗索拉非尼肾毒性的新策略是一项紧迫的工作。钠-葡萄糖共转运蛋白-2 (SGLT2)抑制剂如恩格列净已被批准用于肾衰竭治疗。到目前为止,恩格列净对索拉非尼肾毒性的潜在作用还没有报道。免疫印迹法检测索拉非尼处理肾近端小管细胞(HK-2细胞)中SGLT2和凋亡标志物的表达。用Alamar蓝法测定索拉非尼±恩格列净处理后HK-2细胞的细胞活力。采用免疫印迹法研究索拉非尼±恩格列净对HK-2细胞嗜铁和促炎应激的影响。通过组织病理学分析,研究了接受28天索拉非尼±恩帕列净给药的小鼠肾脏的细胞死亡、铁凋亡、脂质过氧化、细胞因子风暴和免疫细胞募集。索拉非尼暴露剂量依赖性上调HK-2细胞中的SGLT2,恩格列净显著减轻索拉非尼诱导的HK-2细胞和小鼠肾脏细胞死亡。此外,恩格列净显著减轻了索拉非尼刺激的小鼠肾脏铁沉积、氧化DNA损伤、脂质过氧化和谷胱甘肽过氧化物酶4 (GPX4)/ SLC7A11 (xCT)依赖性铁下垂。在HK-2细胞和小鼠肾脏中,索拉非尼促进的环氧化酶-2 (COX-2)/前列腺素E2 (PGE2)信号通路被恩格列清显著阻断。在体外和体内实验中,恩格列净还能减弱索拉非尼刺激的hmgb1 /IL-1β促炎信号。此外,恩格列净显著降低索拉非尼促进的小鼠肾脏巨噬细胞和中性粒细胞浸润。综上所述,恩格列净可以作为一种有效的抗肾衰和抗索拉非尼肾毒性的抗药,通过靶向COX-2/PGE2轴。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


