Arctigenin relieves inflammation and remodels the nasal epithelial barrier function in allergic rhinitis via the KLF5/BIRC3/NFκB axis
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Allergic rhinitis (AR) is a significant global health issue that necessitates effective treatments. This paper investigates the mechanism of arctigenin (ATG) in alleviating AR. An AR mouse model was constructed and administered with different doses of ATG or positive control dexamethasone. Human nasal epithelial cells (HNEpCs) were stimulated with IL-4 and IL-13 to mimic AR-induced epithelial cell damage. Bioinformatic analysis was performed to predict target proteins of ATG and downstream factors of KLF5. AR mice and HNEpCs were treated with KLF5 overexpression lentivirus and BIRC3 knockdown lentivirus. HNEpCs were treated with NF-κB pathway agonist TWEAK. Our results revealed that ATG remodeled the nasal epithelial barrier function and alleviated inflammation in AR mice and inhibited IL-4/IL-13-induced inflammatory injury in HNEpCs. Mechanistically, ATG inhibited the expression of KLF5 protein. KLF5 transcriptionally activated BIRC3 and the NF-κB pathway. KLF5 overexpression exacerbated inflammatory injury in AR mice and HNEpCs, which was reversed by BIRC3 knockdown. NF-κB pathway agonist exacerbated inflammatory injury in HNEpCs. In conclusion, ATG remodels the nasal epithelial barrier function and alleviates AR in mice by inhibiting KLF5 protein expression and BIRC3 transcription and impairing the NF-κB pathway.
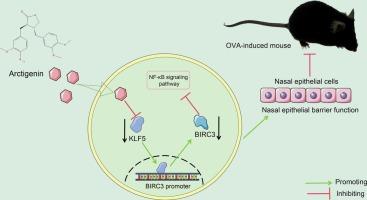
牛蒡子素通过KLF5/BIRC3/NFκB轴缓解变应性鼻炎的炎症并重塑鼻上皮屏障功能
过敏性鼻炎(AR)是一个重要的全球健康问题,需要有效的治疗。本研究探讨了牛角素(ATG)减轻AR的作用机制,建立了AR小鼠模型,并给药不同剂量的ATG或阳性对照地塞米松。用IL-4和IL-13刺激人鼻上皮细胞(HNEpCs),模拟ar诱导的上皮细胞损伤。通过生物信息学分析预测ATG的靶蛋白及KLF5的下游因子。用KLF5过表达慢病毒和BIRC3敲低慢病毒治疗AR小鼠和HNEpCs。应用NF-κB通路激动剂TWEAK治疗HNEpCs。我们的研究结果显示,ATG重塑了AR小鼠的鼻上皮屏障功能,减轻了炎症,抑制了IL-4/ il -13诱导的HNEpCs炎症损伤。机制上,ATG抑制KLF5蛋白的表达。KLF5转录激活BIRC3和NF-κB通路。KLF5过表达加重了AR小鼠和HNEpCs的炎症损伤,而BIRC3敲低可逆转这一过程。NF-κB通路激动剂加重HNEpCs的炎症损伤。综上所述,ATG通过抑制KLF5蛋白表达和BIRC3转录,损害NF-κB通路,重塑小鼠鼻上皮屏障功能,减轻AR。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


