Biallelic MED29 variants cause pontocerebellar hypoplasia with cataracts
IF 4.6
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Pontocerebellar hypoplasia (PCH) represents a group of disorders characterized by cerebellum and pons hypoplasia, variable cerebral involvement, microcephaly, severe global developmental delay (GDD), and seizures. We sought the genetic cause of PCH in two siblings. Genetic workup was performed by whole-exome sequencing followed by Sanger validation. Morpholino-knockdown zebrafish embryos with human wild-type gene rescue were used to assess cerebellar development and motor function. Transfected mouse hippocampal cultures and electroporated mouse embryos were employed to assess functional effects on neuronal morphology and development. Both patients presented with profound GDD, severe microcephaly, cataracts, and variably seizures. Their MRIs demonstrated marked cerebellar and pontine hypoplasia. Both were homozygous for a c.416T > C, p.(Leu139Pro) MED29 variant which was predicted to be pathogenic. Locomotion and cerebellar GABAergic neurons development were both impaired in MED29 Morpholino-knockdown zebrafish and rescued by human wild-type gene expression. ShRNA-knockdown of MED29 in mouse hippocampal neurons decreased neurite length and arborization in vitro, and caused defective embryonic neuronal migration in vivo. Overexpression of MED29 p.(Leu139Pro) was consistent with a loss-of-function. Taken together, the Mediator complex regulates transcription processes, and defects in particular subunits are associated with distinct neurodevelopmental phenotypes involving PCH. We conclude that MED29 is a novel risk gene for PCH.
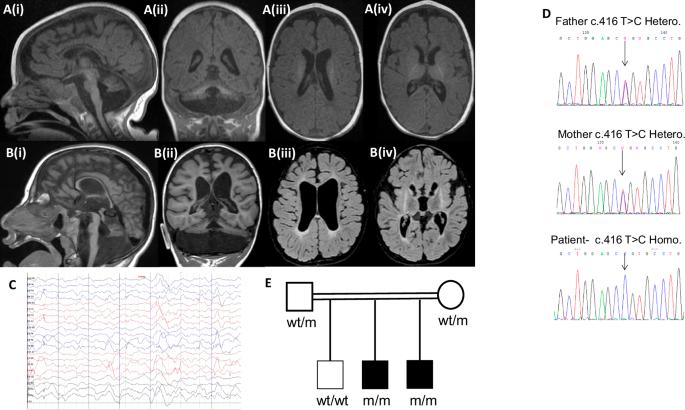
双等位基因MED29变异引起桥小脑发育不全伴白内障。
桥小脑发育不全(PCH)是一组以小脑和桥小脑发育不全、可变脑受累、小头畸形、严重的整体发育迟缓(GDD)和癫痫为特征的疾病。我们在两个兄弟姐妹中寻找PCH的遗传原因。通过全外显子组测序进行遗传检查,然后进行Sanger验证。用人类野生型基因拯救的morpholino敲低斑马鱼胚胎来评估小脑发育和运动功能。采用转染小鼠海马培养物和电穿孔小鼠胚胎来评估功能对神经元形态和发育的影响。两例患者均表现为重度GDD、严重小头畸形、白内障和不同程度的癫痫发作。mri显示明显的小脑和脑桥发育不全。两者都是C . 416t > C, p.(Leu139Pro) MED29变异的纯合子,预计该变异具有致病性。MED29 morpholino敲低斑马鱼的运动和小脑gaba能神经元的发育均受到损害,并被人类野生型基因表达拯救。在体外实验中,敲低小鼠海马神经元中MED29的shrna会减少神经突的长度和树突化,并在体内引起胚胎神经元迁移缺陷。med29p .(Leu139Pro)的过表达与功能丧失一致。综上所述,中介复合物调节转录过程,特定亚基的缺陷与涉及PCH的不同神经发育表型相关。我们认为MED29是PCH的一个新的危险基因。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

European Journal of Human Genetics
生物-生化与分子生物学
CiteScore
9.90
自引率
5.80%
发文量
216
审稿时长
2 months
期刊介绍:
The European Journal of Human Genetics is the official journal of the European Society of Human Genetics, publishing high-quality, original research papers, short reports and reviews in the rapidly expanding field of human genetics and genomics. It covers molecular, clinical and cytogenetics, interfacing between advanced biomedical research and the clinician, and bridging the great diversity of facilities, resources and viewpoints in the genetics community.
Key areas include:
-Monogenic and multifactorial disorders
-Development and malformation
-Hereditary cancer
-Medical Genomics
-Gene mapping and functional studies
-Genotype-phenotype correlations
-Genetic variation and genome diversity
-Statistical and computational genetics
-Bioinformatics
-Advances in diagnostics
-Therapy and prevention
-Animal models
-Genetic services
-Community genetics
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


