Cryo-EM structure of endogenous Plasmodium falciparum Pfs230 and Pfs48/45 fertilization complex
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Malaria parasite fertilization occurs in the midgut of a female Anopheles mosquito. Blocking fertilization within the mosquito can prevent malaria transmission. Plasmodium falciparum Pfs230 and Pfs48/45 proteins are critical for male fertility and transmission of the malaria parasite. They form a core fertilization complex, but it is unknown how they interact. We determined a cryo–electron microscopy structure of the endogenous Pfs230-Pfs48/45 complex showing that Pfs48/45 interacts with Pfs230 domains 13 and 14. Transgenic parasite lines with these domains removed were defective in Pfs230 gamete localization and showed reduced oocyst formation. Nanobodies against domains 13 and 14 inhibited Pfs230-Pfs48/45 complex formation and reduced transmission, and structural analyses revealed their epitopes. These Pfs230 domains were targets of naturally acquired immunity and immune sera from messenger RNA lipid nanoparticle immunizations blocked parasite transmission.
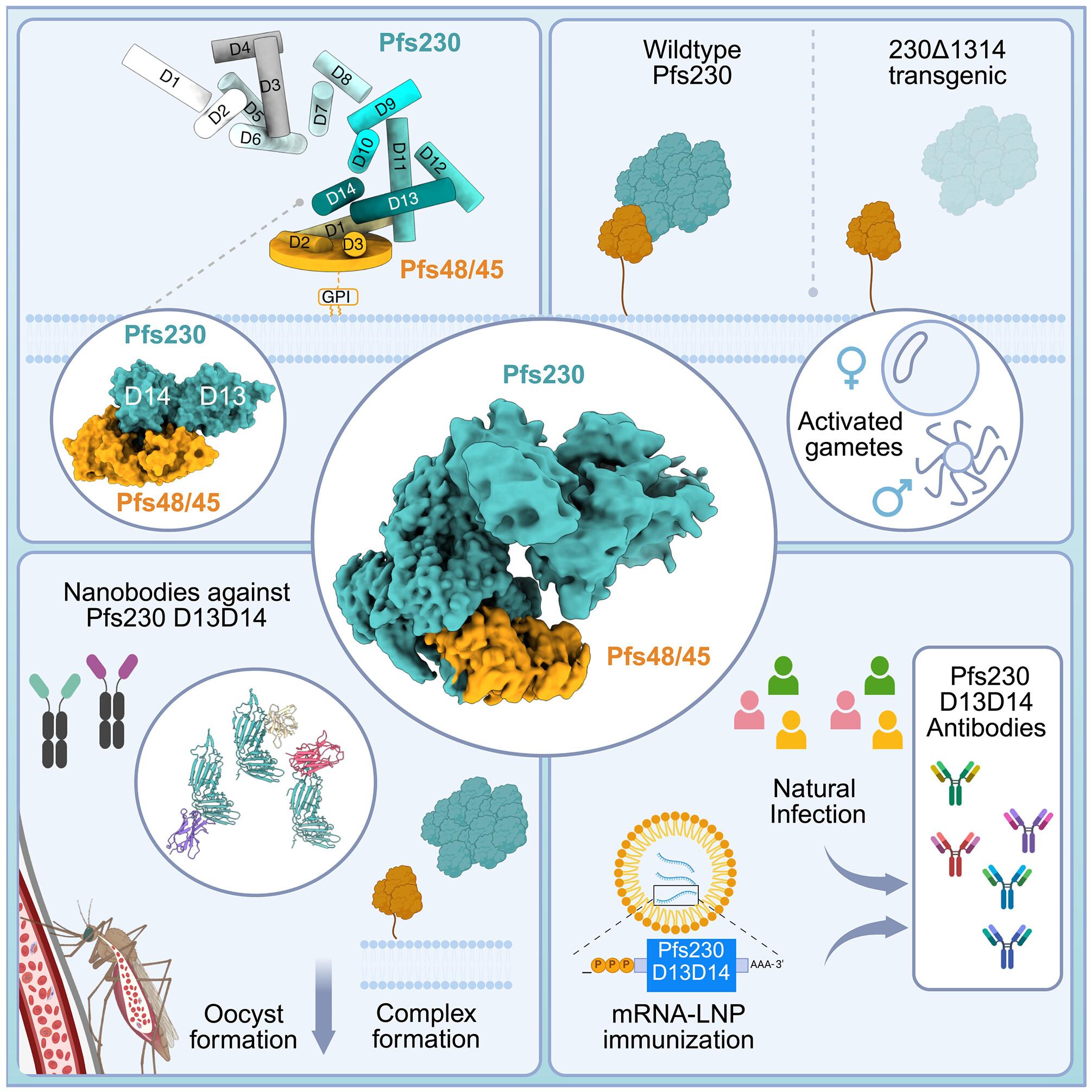
内源性恶性疟原虫Pfs230和Pfs48/45受精复合物的低温电镜结构
疟疾寄生虫受精发生在雌性按蚊的中肠。阻断蚊子体内的受精可以防止疟疾的传播。恶性疟原虫Pfs230和Pfs48/45对男性生育力和疟原虫传播至关重要。它们形成了一个核心受精复合体,但它们是如何相互作用的尚不清楚。我们确定了内源性Pfs230-Pfs48/45复合物的低温电镜结构,显示Pfs48/45与Pfs230结构域13和14相互作用。去除这些结构域的转基因寄生虫系在Pfs230配子定位上存在缺陷,卵囊形成减少。结构域13和14的纳米体抑制了Pfs230-Pfs48/45复合物的形成,减少了传输,结构分析揭示了它们的表位。这些Pfs230结构域是自然获得性免疫的靶点,mrna -脂质纳米颗粒免疫的免疫血清阻断了寄生虫的传播。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


