Inhibition of enzymatic browning in fresh-cut potatoes by 1,3,4-oxadiazole-2-thiol analogs and elucidation of their tyrosinase inhibition mechanism
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Tyrosinase is the rate-limiting enzyme in enzymatic browning, and its activity is closely related to copper ions at the active site. We hypothesized that 1,2,4-oxadiazole derivatives could chelate copper ions at the active site of tyrosinase, thereby inhibiting its activity. Fourteen 1,3,4-oxadiazole-2-thiols were developed to evaluate Cu2+-chelating and anti-tyrosinase effects. Compound 2f (IC50 = 17.7 ± 0.21 μM) had stronger activity than kojic acid (IC50 = 47.7 ± 0.49 μM) as a mixed-type inhibitor. HPLC revealed that 2f inhibited dopaquinone formation, whereas UV/CD spectroscopy showed that 2f induced tyrosinase conformational changes. Fluorescence quenching, Cu2+ chelation, and molecular docking analyses confirmed the direct Cu2+-binding ability of 2f. In addition, 2f had low cytotoxicity and effectively prevented browning in fresh-cut potatoes. These results confirm our hypothesis that thiol-containing 1,3,4-oxadiazole derivatives inhibit tyrosinase for Cu2+ chelation and hydrophobic interactions. The results support their potential as safe, efficient anti-browning agents for fresh-cut produce preservation, addressing industry needs for practical enzymatic browning control.
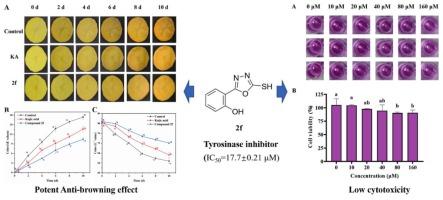
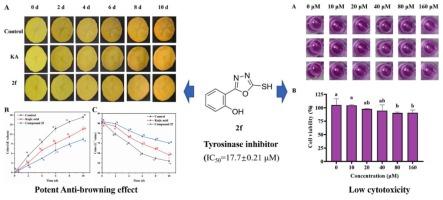
1,3,4-恶二唑-2-硫醇类似物对鲜切马铃薯酶促褐变的抑制作用及其酪氨酸酶抑制机制的阐明
酪氨酸酶是酶促褐变的限速酶,其活性与活性位点的铜离子密切相关。我们假设1,2,4-恶二唑衍生物可以在酪氨酸酶的活性位点螯合铜离子,从而抑制其活性。开发了14个1,3,4-恶二唑-2-硫醇来评估Cu2+螯合和抗酪氨酸酶的作用。化合物2 f (IC50 = 17.7±0.21 μM)比曲酸更强的活动(IC50 = 47.7±0.49 μM)混合型缓蚀剂。HPLC显示2 f抑制多巴醌的形成,而UV/CD光谱显示2 f诱导酪氨酸酶构象改变。荧光猝灭、Cu2+螯合和分子对接分析证实了2 f的Cu2+直接结合能力。此外,2 f具有较低的细胞毒性,可有效防止鲜切土豆的褐变。这些结果证实了我们的假设,即含硫醇的1,3,4-恶二唑衍生物抑制酪氨酸酶的Cu2+螯合和疏水相互作用。结果支持他们的潜力作为安全,有效的抗褐变剂鲜切农产品保存,解决实际的酶促褐变控制工业需求。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


