Significant enhancement of inhibitory strength of bisphenol A analogs against human and rat 5α-reductase 1 upon halogenation of the benzene ring: Potentially disrupting neurosteroid biosynthesis
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Bisphenol A (BPA) halogenation derivatives, formed via radical or electrophilic substitution, constitute a class of emerging contaminants, with brominated variants dominating the flame-retardant market. Their effect on steroid 5α-reductase 1 (SRD5A1) activity in neural and testicular cells remains unclear. This study examined inhibitory effects of seven BPA analogs on SRD5A1, focusing on dihydrotestosterone synthesis. Using SRD5A1 in human brain SF126 cell and rat testicular microsomes, we found an inhibitory potency gradient. For human SRD5A1: tetrabromobisphenol A (12.71 μM) > tetrachlorobisphenol A (16.01 μM) > trichlorobisphenol A (18.95 μM) > 3-chlorobisphenol A (45.37 μM) > BPA = bisphenol S (BPS) = tetrabromobisphenol S (no effect at 100 μM). For rat enzyme: tetrabromobisphenol A (3.60 μM) > tetrachlorobisphenol A (9.27 μM) > trichlorobisphenol A (12.43 μM) > 3-chlorobisphenol A (26.37 μM) > BPA (over 100 μM) = BPS (over 100 μM) > tetrabromobisphenol S (no effect at 100 μM). The inhibition was mixed/competitive. Structure-activity relationship (SAR) analysis showed negative correlations between IC50 values and logarithm of the partition coefficient (LogP), heavy atoms, and hetero atoms of these chemicals for both enzymes. 3D-QSAR pharmacophore analysis showed key binding features to human SRD5A1. Tetrabromobisphenol A has all, while 3-chlorobisphenol A only has hydrophobic sites. Computational docking explained molecular interactions with SRD5A1 binding sites, linking to physicochemical properties. These findings demonstrate SRD5A1 inhibition nuances by halogenated BPA analogs with direct relevance to potential implications. Our data establish the inhibitory potential of halogenated BPA analogs on human brain SRD5A1, revealing distinct SAR and molecular interactions. The inclusion of the rat model provided comparative insight into species-specific responses, underscoring the importance of considering both human relevance and interspecies variability in evaluating endocrine-disrupting risks.
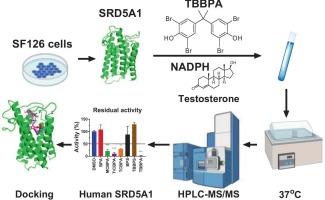
双酚A类似物在苯环卤化后对人和大鼠5α-还原酶1的抑制强度显著增强:可能破坏神经类固醇的生物合成。
通过自由基或亲电取代形成的双酚A (BPA)卤化衍生物构成了一类新兴污染物,其中溴化变体主导着阻燃剂市场。它们对神经细胞和睾丸细胞中类固醇5α-还原酶1 (SRD5A1)活性的影响尚不清楚。本研究考察了7种双酚a类似物对SRD5A1的抑制作用,重点研究了双氢睾酮的合成。利用SRD5A1对人脑SF126细胞和大鼠睾丸微粒体的抑制作用,我们发现了一个抑制效价梯度。对人类SRD5A1:四溴双酚A(12.71 μM) > tetrachlorobisphenol一(16.01 μM) > trichlorobisphenol一(18.95 μM) > 3-chlorobisphenol一(45.37 μM) > BPA = 双酚S (BPS) = tetrabromobisphenol年代(100年没有影响 μM)。老鼠酶:四溴双酚A(3.60 μM) > tetrachlorobisphenol一(9.27 μM) > trichlorobisphenol一(12.43 μM) > 3-chlorobisphenol一(26.37 μM) > BPA(超过100 μM) = BPS(超过100 μM) > tetrabromobisphenol年代(100年没有影响 μM)。抑制是混合的/竞争性的。构效关系(SAR)分析表明,IC50值与配分系数(LogP)、重原子和杂原子的对数呈负相关。3D-QSAR药效团分析显示了与人SRD5A1的关键结合特征。四溴双酚A有所有的,而3-氯双酚A只有疏水位点。计算对接解释了分子与SRD5A1结合位点的相互作用,连接到物理化学性质。这些发现表明,卤化双酚a类似物对SRD5A1的抑制存在细微差别,与潜在的影响直接相关。我们的数据建立了卤代双酚a类似物对人脑SRD5A1的抑制潜力,揭示了不同的SAR和分子相互作用。纳入大鼠模型提供了对物种特异性反应的比较见解,强调了在评估内分泌干扰风险时考虑人类相关性和物种间变异性的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


