Characterizing primary and secondary senescence in vivo
IF 19.4
Q1 CELL BIOLOGY
引用次数: 0
Abstract
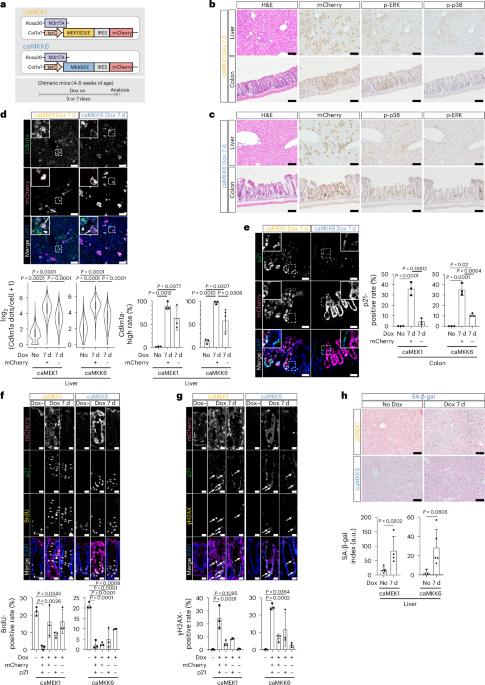
There is robust evidence that senescence can be propagated in vitro through mechanisms including the senescence-associated secretory phenotype, resulting in the non-cell-autonomous induction of secondary senescence. However, the induction, regulation and physiological role of secondary senescence in vivo remain largely unclear. Here we generated senescence-inducible mouse models expressing either the constitutively active form of MEK1 or MKK6 and mCherry, to map primary and secondary senescent cells. Our models recapitulate characteristic features of senescence and demonstrate that primary and secondary phenotypes are highly tissue- and inducer-dependent. Spatially resolved RNA expression analyses at the single-cell level reveal that each senescence induction results in a unique transcriptional profile—even within cells of the same cell type—explaining the heterogeneity of senescent cells in vivo. Furthermore, we show that interleukin-1β, primarily derived from macrophages, induces secondary phenotypes. Our findings provide insight into secondary senescence in vivo and useful tools for understanding and manipulating senescence during aging. Through non-cell-autonomous functions, senescent cells can induce secondary senescence in bystander cells. Sogabe et al. generate mouse models of MEK1- or MKK6-induced senescence and use double labeling to map primary and secondary senescence in vivo using single-cell and spatial analysis.
体内原发性和继发性衰老的特征。
有强有力的证据表明,衰老可以通过包括衰老相关分泌表型在内的机制在体外繁殖,从而导致非细胞自主诱导的继发性衰老。然而,体内继发性衰老的诱导、调控和生理作用仍不清楚。在这里,我们建立了衰老诱导小鼠模型,表达MEK1或MKK6和mCherry的组成活性形式,以绘制原发性和继发性衰老细胞。我们的模型概括了衰老的特征,并证明初级和次级表型高度依赖于组织和诱导剂。单细胞水平的空间分辨RNA表达分析表明,即使在同一细胞类型的细胞内,每种衰老诱导都会导致独特的转录谱,这解释了体内衰老细胞的异质性。此外,我们发现主要来源于巨噬细胞的白细胞介素-1β可诱导继发性表型。我们的研究结果为理解和控制衰老过程中的衰老提供了有用的工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


