Identification of transmembrane channel–like 4-targeting saltiness–enhancing peptides derived from chicken skin collagen and evaluation of their antioxidant abilities
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Saltiness–enhancing peptides are a novel class of salt substituents capable of partially replacing sodium chloride (NaCl) in food products. The current study describes the isolation and identification of saltiness–enhancing peptides from chicken skin collagen hydrolysate. Six of the peptides, including GERGPKGPKGETGPT (GT15), GPAGPPGARGQAG (GG13), GPPGPTGPA (GA9), GPPGPTGPAGER (GR12), VGPTGPAGPR (VR10), and VGSP (VP4), were found to enhance the saltiness intensity of a 0.35 % (w/v) solution of NaCl. These six peptides exhibited an umami taste, with GT15 and GR12 additionally having a salty taste. Molecular dynamics simulation revealed that these peptides can stably bind to the salty taste receptor transmembrane channel–like 4 via hydrogen bonds. In vitro studies revealed that these peptides exhibited antioxidant activities. These six saltiness–enhancing peptides can therefore be potentially applied for augmenting the saltiness of NaCl solution and partially replacing salt in food products while preventing the oxidative damage typically caused by high amounts of salt.
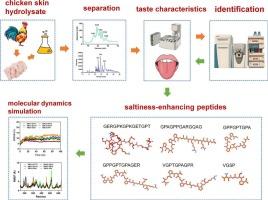
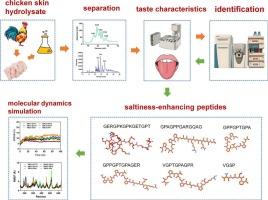
鸡皮肤胶原蛋白跨膜通道样4-靶向盐性增强肽的鉴定及其抗氧化能力评价
咸味增强肽是一类新的盐取代基,能够部分取代食品中的氯化钠(NaCl)。本研究描述了从鸡皮肤胶原蛋白水解物中分离和鉴定盐分增强肽的方法。其中,GERGPKGPKGETGPT (GT15)、GPAGPPGARGQAG (GG13)、GPPGPTGPA (GA9)、GPPGPTGPAGER (GR12)、VGPTGPAGPR (VR10)和VGSP (VP4)等6个多肽可增强0.35 % (w/v) NaCl溶液的咸味强度。这六种肽具有鲜味,其中GT15和GR12具有咸味。分子动力学模拟表明,这些肽可以通过氢键稳定地结合到咸味受体的跨膜通道4上。体外研究表明,这些肽具有抗氧化活性。因此,这六种增盐肽可以潜在地应用于提高NaCl溶液的咸度和部分替代食品中的盐,同时防止通常由大量盐引起的氧化损伤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


