Cerebellar circuit computations for predictive motor control
IF 26.7
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
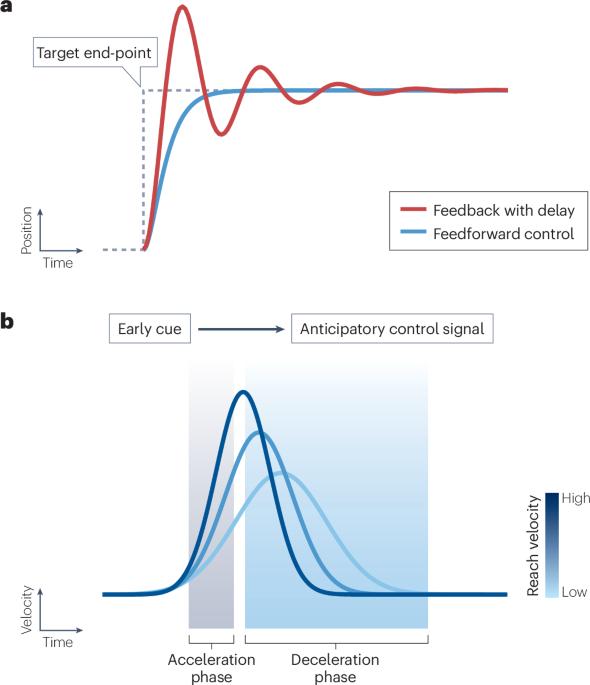
The rise of the deep neural network as the workhorse of artificial intelligence has brought increased attention to how network architectures serve specialized functions. The cerebellum, with its largely shallow, feedforward architecture, provides a curious example of such a specialized network. Within the cerebellum, tiny supernumerary granule cells project to a monolayer of giant Purkinje neurons that reweight synaptic inputs under the instructive influence of a unitary synaptic input from climbing fibres. What might this predominantly feedforward organization confer computationally? Here we review evidence for and against the hypothesis that the cerebellum learns basic associative feedforward control policies to speed up motor control and learning. We contrast and link this feedforward control framework with another prominent set of theories proposing that the cerebellum computes internal models. Ultimately, we suggest that the cerebellum may implement control through mechanisms that resemble internal models but involve model-free implicit mappings of high-dimensional sensorimotor contexts to motor output. The cerebellum helps ensure the speed and accuracy of movements, but its precise contributions to movement control are unclear. Nguyen and Person here evaluate evidence for and against feedforward motor control by the cerebellum in light of its well-defined role in a model of associative learning, and reconcile this with theories of internal model-based control.
预测运动控制的小脑电路计算
作为人工智能的主力,深度神经网络的兴起引起了人们对网络架构如何服务于特定功能的关注。小脑具有很大的浅层前馈结构,为这种特殊的网络提供了一个奇怪的例子。在小脑内,微小的多余颗粒细胞投射到巨大的浦肯野神经元的单层上,在来自攀爬纤维的单一突触输入的指导影响下,这些神经元重新计算突触输入的权重。这种以前馈为主的组织在计算上可能会赋予我们什么?在这里,我们回顾了支持和反对小脑学习基本联想前馈控制策略以加速运动控制和学习的假设的证据。我们将这种前馈控制框架与另一套提出小脑计算内部模型的著名理论进行对比和联系。最后,我们认为小脑可能通过类似于内部模型的机制来实现控制,但涉及高维感觉运动上下文到运动输出的无模型内隐映射。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Neuroscience
NEUROSCIENCES-
自引率
0.60%
发文量
104
期刊介绍:
Nature Reviews Neuroscience is a multidisciplinary journal that covers various fields within neuroscience, aiming to offer a comprehensive understanding of the structure and function of the central nervous system. Advances in molecular, developmental, and cognitive neuroscience, facilitated by powerful experimental techniques and theoretical approaches, have made enduring neurobiological questions more accessible. Nature Reviews Neuroscience serves as a reliable and accessible resource, addressing the breadth and depth of modern neuroscience. It acts as an authoritative and engaging reference for scientists interested in all aspects of neuroscience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


