ACE-inhibitory peptides from Morchella esculenta: screening, kinetics, and molecular dynamics simulation
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
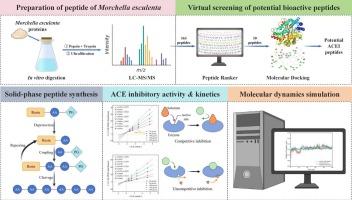
The global prevalence of hypertension has doubled over the past three decades and it is projected to escalate further. Hypertension is a global health concern that is closely linked to angiotensin-converting enzyme (ACE) regulation. Conventional ACE inhibitors demonstrate clinical efficacy; however, their frequent adverse effects underscore an urgent demand for safer therapeutic alternatives. In this context, our study investigates Morchella esculenta as a potential natural source of ACE-inhibitory peptides. The <3 kDa fraction was identified as exhibiting the highest inhibitory activity through the systematic screening of hydrolysates across multiple molecular weight ranges. HPLC-MS/MS analysis identified 163 peptides, of which five were selected for further experiments. Solid-phase synthesis confirmed that LIVPSLPGYAF exhibited the strongest ACE inhibition (IC50 = 50.99 μM). Inhibition kinetics showed LIVPSLPGYAF acted as a mixed-type inhibitor, while GLGPLAQLIWDR and LIFHSFGGTGSGF functioned as competitive inhibitors. Molecular dynamics simulations validated their stable binding to the ACE complex. These findings suggested that Morchella esculenta is a natural source of ACE inhibitory peptides and can potentially be used as a component in functional foods for the treatment of hypertension.
羊肚菌ace抑制肽:筛选、动力学和分子动力学模拟
在过去三十年中,全球高血压患病率翻了一番,预计还会进一步上升。高血压是一个与血管紧张素转换酶(ACE)调节密切相关的全球性健康问题。常规ACE抑制剂显示临床疗效;然而,它们频繁的副作用强调了对更安全的治疗替代品的迫切需求。在这种情况下,我们的研究调查羊肚菌作为ace抑制肽的潜在天然来源。通过系统筛选多个分子量范围内的水解产物,鉴定出<;3 kDa片段具有最高的抑制活性。HPLC-MS/MS分析鉴定出163个多肽,其中5个被选择用于进一步的实验。固相合成证实LIVPSLPGYAF具有最强的ACE抑制作用(IC50 = 50.99 μM)。抑制动力学表明LIVPSLPGYAF是混合型抑制剂,GLGPLAQLIWDR和LIFHSFGGTGSGF是竞争性抑制剂。分子动力学模拟验证了它们与ACE复合物的稳定结合。这些发现表明羊肚菌是ACE抑制肽的天然来源,可以作为治疗高血压的功能性食品的成分。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


