Near-Field Terahertz Nanoscopy Spatially Resolves Chiral Drug–Cell Interactions: Toward Precision Intracellular Pharmacology
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Chiral drug enantiomers frequently exhibit marked differences in pharmacological efficacy and toxicity profiles. However, the resolution limitations of traditional detection methodologies render them incapable of resolving the spatial heterogeneity of drug distribution within individual cells and obscure our understanding of subcellular drug distribution and mechanism of action. Here, we employ terahertz scattering-type scanning near-field optical microscopy (THz s-SNOM), a cutting-edge technique offering nanoscale spatial resolution and label-free imaging capabilities, to investigate single-cell response heterogeneity to chiral drug exposure. We systematically analyzed morphological and biochemical compositional differences between control cells and those treated with RS-ibuprofen, (R)-(−)-ibuprofen, and (S)-(+)-ibuprofen. Our high-resolution analysis revealed that the mean area of plasma membrane-derived extracellular vesicles (PEVs) in drug-treated cells increased by 24.6%, 25.4%, and 39.9%, respectively, compared to the representative control cell. Through principal component analysis and Euclidean distance distribution methods, we identified distinct spectral variations in both intracellular and nuclear regions. Notably, these three enantiomeric forms induced fundamentally different patterns of heterogeneous evolution within cellular compartments. RS-ibuprofen treatment specifically resulted in increased membrane heterogeneity concomitant with decreased nuclear heterogeneity. Quantitative analysis of spectral intensity differences within nuclear regions (p < 0.001), based on single-frequency imaging and clustering analyses at characteristic THz peak frequencies, provided direct evidence of drug-induced heterogeneity. These findings establish a novel paradigm for mapping drug–cell interactions at the nanoscale, conceptualizing these processes as spatially encoded phenomena, thereby advancing the field of subcellular chiral pharmacology.
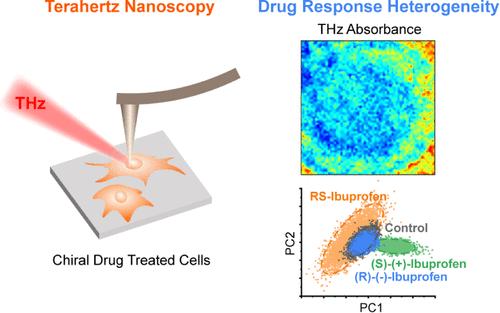
近场太赫兹纳米镜在空间上解析手性药物-细胞相互作用:迈向精确的细胞内药理学
手性药物对映体在药理功效和毒性方面经常表现出明显的差异。然而,传统检测方法的分辨率限制使得它们无法解决单个细胞内药物分布的空间异质性,并且模糊了我们对亚细胞药物分布和作用机制的理解。在这里,我们采用太赫兹散射型扫描近场光学显微镜(THz s-SNOM),一种提供纳米级空间分辨率和无标记成像能力的尖端技术,来研究单细胞对手性药物暴露的反应异质性。我们系统地分析了对照细胞与经RS-ibuprofen、(R)-(−)-ibuprofen和(S)-(+)-ibuprofen处理的细胞在形态和生化组成上的差异。我们的高分辨率分析显示,与代表性对照细胞相比,药物处理细胞的质膜来源的细胞外囊泡(PEVs)的平均面积分别增加了24.6%,25.4%和39.9%。通过主成分分析和欧几里得距离分布方法,我们确定了细胞内和核区明显的光谱变化。值得注意的是,这三种对映体形式在细胞区室内诱导了根本不同的异质进化模式。rs -布洛芬治疗特别导致膜非均质性增加,同时核非均质性降低。核区光谱强度差异定量分析(p <;0.001),基于单频成像和特征太赫兹峰频率的聚类分析,提供了药物诱导异质性的直接证据。这些发现为在纳米尺度上绘制药物-细胞相互作用图谱建立了一个新的范例,将这些过程概念化为空间编码现象,从而推动了亚细胞手性药理学领域的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


