Impact of partial enzymatic hydrolysis on the transport and uptake of SPI nanoparticles post-digestion
IF 5.1
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
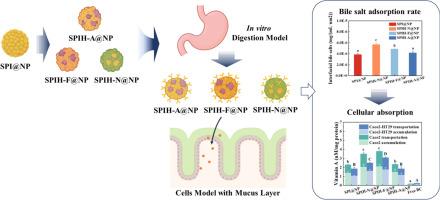
Despite extensive research on protein-based nanoparticles for nutrient delivery, the structural features that govern bile salt interactions and subsequently enhance intestinal absorption and metabolic transformation remain insufficiently clarified. In this study, soy protein isolate nanoparticles enzymatically modified by Alcalase, Neutrase, and Flavorzyme (SPIH-A@NP, SPIH![]() N@NP, SPIH-F@NP) were constructed as structural models to explore the relationship between nanoparticle interfacial properties, bile salt adsorption, and nutrient transport efficiency, using simulated gastrointestinal digestion and Caco-2/HT29 co-culture models. Compared with undigested nanoparticles and free β-carotene, digested nanoparticles exhibited significantly improved cellular uptake and transport efficiencies. Among them, SPIH
N@NP, SPIH-F@NP) were constructed as structural models to explore the relationship between nanoparticle interfacial properties, bile salt adsorption, and nutrient transport efficiency, using simulated gastrointestinal digestion and Caco-2/HT29 co-culture models. Compared with undigested nanoparticles and free β-carotene, digested nanoparticles exhibited significantly improved cellular uptake and transport efficiencies. Among them, SPIH![]() N@NP and SPIH-F@NP retained more hydrophobic peptides and acidic subunits from 11S globulin, which enhanced bile salt adsorption, maintained smaller particle sizes post-digestion, and facilitated more efficient delivery of β-carotene and its metabolites. Additionally, the digestion process altered uptake pathways, with macropinocytosis contributing more prominently post-digestion. These findings provide mechanistic insight into structure–function relationships that can guide the rational design of protein-based nanocarriers for targeted absorption and metabolic modulation of lipophilic nutrients.
N@NP and SPIH-F@NP retained more hydrophobic peptides and acidic subunits from 11S globulin, which enhanced bile salt adsorption, maintained smaller particle sizes post-digestion, and facilitated more efficient delivery of β-carotene and its metabolites. Additionally, the digestion process altered uptake pathways, with macropinocytosis contributing more prominently post-digestion. These findings provide mechanistic insight into structure–function relationships that can guide the rational design of protein-based nanocarriers for targeted absorption and metabolic modulation of lipophilic nutrients.
部分酶解对消化后SPI纳米颗粒运输和摄取的影响
尽管基于蛋白质的纳米颗粒用于营养输送的研究广泛,但控制胆盐相互作用并随后增强肠道吸收和代谢转化的结构特征仍未得到充分阐明。本研究通过模拟胃肠道消化和Caco-2/HT29共培养模型,构建Alcalase、Neutrase和Flavorzyme (SPIH-A@NP, SPIHN@NP, SPIH-F@NP)酶修饰的大豆分离蛋白纳米颗粒作为结构模型,探讨纳米颗粒界面特性、胆盐吸附和营养物质运输效率之间的关系。与未消化的纳米颗粒和游离β-胡萝卜素相比,消化的纳米颗粒表现出显著提高的细胞摄取和运输效率。其中SPIHN@NP和SPIH-F@NP从11S球蛋白中保留了更多的疏水肽和酸性亚基,增强了胆盐的吸附,在消化后保持更小的粒径,促进了β-胡萝卜素及其代谢物的更有效递送。此外,消化过程改变了摄取途径,消化后巨噬细胞增多作用更为突出。这些发现提供了结构-功能关系的机制见解,可以指导基于蛋白质的纳米载体的合理设计,以靶向吸收和亲脂性营养物质的代谢调节。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


