Cross-reactivity and conserved epitope analysis of tropomyosin from Lateolabrax japonicus and shellfish species
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The phenomenon of cross-reactivity within TM between fish and shellfish has received scant attention. In the study, TM of Lateolabrax japonicus, Scylla paramamosain and Mactra quadrangularis were considered as research objects. The results indicated L. japonicus TM exhibited weak binding for both rabbit anti-S. paramamosain/M. quadrangularis TM antibody. However, a significant IgE-binding capacity was observed for three TM in 11 of 18 fish-sensitised patients. Additionally, three TM also could activated basophils in 6 fish-sensitised patients. Subsequently, seven IgE epitopes of L. japonicus TM were confirmed. Two conserved epitopes (LERTEERA, LKTVQNN) and four critical amino acids (E, A, L, and R) were found across L. japonicus and shellfish TM, suggesting these could be responsible for cross-reactivity. These findings were expected to reduce the allergenicity of fish by destroying IgE epitopes through food processing or deleting IgE epitopes using molecular biotechnology, which have important implications for food safety and allergy management.
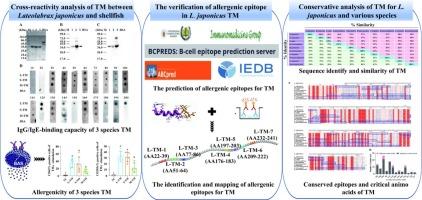
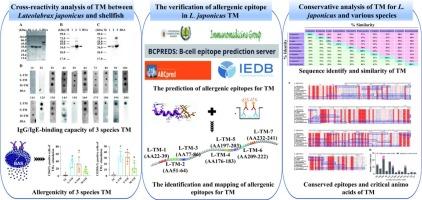
日本鳗鲡与贝类原肌球蛋白的交叉反应性及保守表位分析
鱼类和贝类在TM内的交叉反应现象很少受到关注。在研究中,以日本刺槐(Lateolabrax japonicus)的TM、Scylla paramamosain和Mactra quadrangularis为研究对象。结果表明,日本松菌TM对兔抗s和s均有较弱的结合。paramamosain / M。quadrangularis TM抗体。然而,在18例鱼致敏患者中,有11例观察到3种TM具有显著的ige结合能力。此外,3种TM还能激活6例鱼致敏患者的嗜碱性细胞。随后,确定了日本血吸虫TM的7个IgE表位。两个保守的表位(LERTEERA, LKTVQNN)和4个关键氨基酸(E, A, L, R)在L. japonicus和贝类TM中均被发现,表明它们可能与交叉反应有关。这些发现有望通过食品加工破坏IgE表位或利用分子生物技术去除IgE表位来降低鱼类的致敏性,这对食品安全和过敏管理具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


