Exploring the impact of processing temperatures on cod protein modifications by α, β-unsaturated aldehydes using a clickable probe
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
To investigate the effects of processing temperatures on α, β-unsaturated aldehyde-mediated modifications of cod protein, a clickable probe, 4-(2-Propyn-1-yloxy)-2-butenal (yne-ACR), was developed to simulate α, β-unsaturated aldehyde modifications on cod protein in simulated processing systems (25 °C, 90 °C, and 180 °C). Processing at 180 °C resulted in a 3-fold increase in the hydrophobicity, while modifications under thermal processing had limited effects on the content of free nucleophilic amino acids. However, modification at 180 °C significantly increased the content of carbonylated proteins to 14.7 nmol/mg protein. Cod protein modified during thermal processing (90 °C and 180 °C) exhibited superior emulsifying and foaming properties than those subjected to heating alone. Cod protein processed at 90 °C exhibited 757 identified modified components, representing the highest number among the three processing temperatures, and thermally induced structural changes in cod protein facilitated multiple modifications on a single peptide. These findings provide new tools and insights for the study of food protein modification.
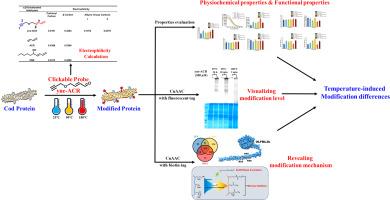
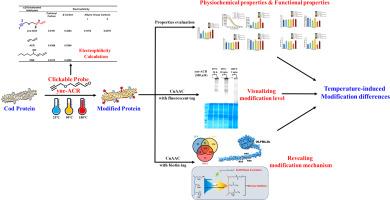
利用可点击探针探索加工温度对α, β-不饱和醛修饰鳕鱼蛋白的影响
为了研究加工温度对α, β-不饱和醛介导的鳕鱼蛋白修饰的影响,开发了一个可点击探针,4-(2-丙基-1-yloxy)-2-丁烯醛(炔- acr),以模拟α, β-不饱和醛在模拟加工系统(25 °C, 90 °C和180 °C)中对鳕鱼蛋白的修饰。在180 °C下处理导致疏水性增加3倍,而热处理下的改性对游离亲核氨基酸含量的影响有限。然而,180 °C的修饰显著提高了羰基化蛋白的含量,达到14.7 nmol/mg蛋白。经过热处理(90 °C和180 °C)改性的鳕鱼蛋白比单独加热的鳕鱼蛋白表现出更好的乳化和发泡性能。在90 °C下加工的鳕鱼蛋白显示了757个已鉴定的修饰成分,是三种加工温度中最多的,并且热诱导的鳕鱼蛋白结构变化有利于在单个肽上进行多重修饰。这些发现为食品蛋白改性的研究提供了新的工具和见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


