Effect of salt extraction on composition, structure, and thermal properties of pea protein
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Salt plays a vital role in modulating protein solubility during plant protein extraction. This study investigated the effect of salt concentrations during extraction on the composition, structure, and thermal properties of pea protein extracts. Low concentrations (0.0–0.2 M) of NaCl resulted in higher ratio of convicilin and vicilin with higher molar mass. Salt concentration did not affect the molar mass of legumin. With 0.4 M NaCl, protein extractability peaked at 78 % and extracted protein had the highest legumin-to-vicilin ratio. With NaCl concentrations greater than 0.4 M, protein composition of extracts remained unchanged, but the extractability decreased. Salt enhanced the heat stability of all pea proteins, as measured by NanoDSC. This study demonstrated that varying NaCl concentrations during protein extraction resulted in pea proteins with different compositions, structures, and thermal properties, offering valuable insights for developing customized protein extraction. The findings can also be extended to other plant proteins.

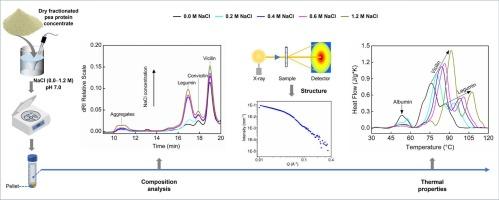
盐萃取对豌豆蛋白组成、结构和热性能的影响
在植物蛋白提取过程中,盐对蛋白质溶解度的调节起着至关重要的作用。本研究考察了提取过程中不同盐浓度对豌豆蛋白提取物的组成、结构和热性能的影响。较低浓度(0.0 ~ 0.2 M) NaCl可使服毒素与维西林的比值增大,摩尔质量增大。盐浓度对豆豆的摩尔质量没有影响。当NaCl浓度为0.4 M时,蛋白质提取率最高,达到78 %,提取的蛋白质中豆科蛋白与维西蛋白的比值最高。NaCl浓度大于0.4 M时,提取物的蛋白质组成基本不变,但提取率下降。通过NanoDSC测量,盐增强了所有豌豆蛋白的热稳定性。该研究表明,在蛋白质提取过程中,不同的NaCl浓度会导致豌豆蛋白质具有不同的组成、结构和热性能,为开发定制蛋白质提取提供了有价值的见解。这一发现也可以推广到其他植物蛋白。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


