Separation and Detection of Charged Unilamellar Vesicles in Vacuum by a Frequency-Controlled Quadrupole Mass Sensor
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
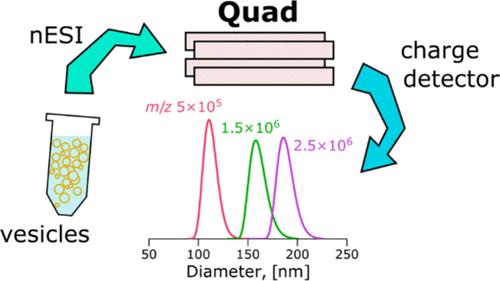
Extracellular vesicles (EVs) are membranous particles released by cells and are considered to be promising sources of biomarkers for various diseases. Mass spectrometry (MS) analysis of EVs requires a sample of purified and detergent-lysed EVs. Purification of EVs is laborious, based on size, density, or surface nature, and requires large amounts of the source material (e.g., blood, spinal fluid). We have employed synthetically produced large unilamellar lipid vesicles (LUVs) as analogs of EVs to demonstrate an alternative approach to vesicle separation for subsequent mass spectrometry analysis of their composition. Mass-to-charge ratio m/z separation by frequency-controlled quadrupole was employed to filter narrow-size distributions of LUVs from a water sample. Lipid vesicles were positively charged with nanoelectrospray and transferred into a vacuum using two wide m/z-range frequency-controlled quadrupoles. The m/z, charges, and masses of individual vesicles were obtained by the nondestructive single-pass charge detector. The resolving mode of the second quadrupole with m/z RSD < 10% allowed to separate size selected distributions of vesicles with modal diameters of 88, 112, 130, 162, and 190 nm at corresponding quadrupole m/z settings of 2.5 × 105, 5 × 105, 8 × 105, 1.5 × 106, and 2.5 × 106, respectively with a rate of 20–100 counts per minute. The distributions of bioparticles with masses between 108 and 1010 Da were separated from human blood serum in the pilot experiment. The presented approach for lipid vesicle separation encourages the development of new techniques for the direct mass-spectrometric analysis of biomarkers in MS-separated EVs in a vacuum.
利用频控四级杆质量传感器分离和检测真空中的带电单拉米质囊泡
细胞外囊泡(EVs)是细胞释放的膜状颗粒,被认为是多种疾病生物标志物的有前途的来源。电动汽车的质谱(MS)分析需要纯化和洗涤剂裂解的电动汽车样本。基于尺寸、密度或表面性质,电动汽车的纯化是费力的,并且需要大量的源材料(例如血液、脊髓液)。我们采用人工合成的大单层脂质囊泡(LUVs)作为ev的类似物,以证明一种替代囊泡分离的方法,用于随后的质谱分析其成分。采用频率控制四极杆的质量-电荷比m/z分离,从水样中过滤出窄尺寸的luv分布。脂质囊泡被纳米电喷雾带正电荷,并通过两个宽m/z范围的变频四极杆转移到真空中。单个囊泡的m/z、电荷和质量由非破坏性单次电荷检测器获得。具有m/z RSD <的第二四极子解析模式10%允许分离模态直径为88、112、130、162和190 nm的囊泡的大小选择分布,在相应的四极杆m/z设置分别为2.5 × 105、5 × 105、8 × 105、1.5 × 106和2.5 × 106,速率为每分钟20-100次。从人血清中分离出质量在108 ~ 1010da之间的生物颗粒。提出的脂质囊泡分离方法促进了真空中ms分离ev生物标志物直接质谱分析新技术的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


