Function Identification of a Lactonase Gene lac1563 Involved in Producing Urolithin A of Limosilactobacillus fermentum FUA033
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
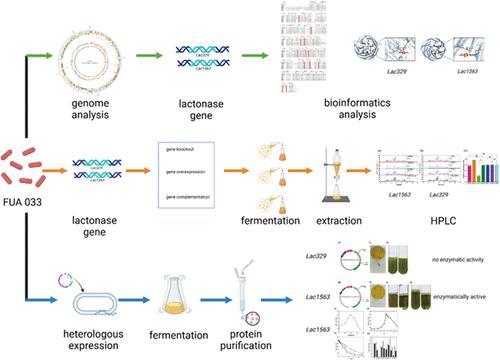
This study aimed to identify the lactonase gene in Limosilactobacillus fermentum FUA033 that facilitates the conversion of ellagic acid into urolithin A. Lactonase gene candidates were identified genome analysis. A series of overexpression, knockout, and complementation strains of these candidate genes were subsequently generated. The urolithin A yield of the lac1563 knockout strain was significantly reduced compared to the wild-type strain, whereas the overexpression strain exhibited a markedly higher yield. The complementation strain showed no significant difference in urolithin A yield relative to the wild-type strain. In contrast, alterations in the lac329 gene did not significantly impact urolithin A production compared to the wild-type strain. Additionally, the candidate lactonase genes were cloned and heterologously expressed in Escherichia coli. The recombinant lactonase derived from the lac1563 gene, unlike that from the lac329 gene, demonstrated activity toward ellagic acid. Optimal activity of the lac1563 lactonase occurred at 37 °C and pH 7.0. Enzyme activity was notably enhanced by Mn2+ and Co2+, while inhibited by Zn2+ and Cu2+. These findings contribute valuable insights into the metabolic pathway of ellagic acid conversion to urolithin A and establish the lac1563 gene as a promising candidate for biotechnological applications aimed at urolithin A production.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


