Electrochemical Sensing of Perfluorooctanoic Acid via a Rationally Designed Fluorine-Functionalized Cu-MOF and In-Depth Analysis of Sensing Mechanism
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
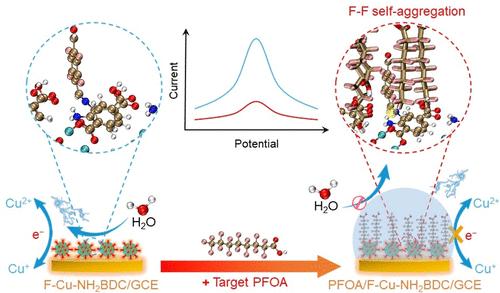
Perfluorooctanoic acid (PFOA), a prominent member of the per- and polyfluoroalkyl substance (PFAS) family, has emerged as a new perpetual pollutant posing significant environmental and health risks, necessitating developing highly selective materials for its sensitive detection in water. In this work, we developed an electroactive fluorine-functionalized Cu-MOF (F–Cu–NH2BDC) through postmodification of the copper-2-amino-terephthalic acid (Cu–NH2BDC) MOF with 2,3,5,6-tetrafluoroterephthalaldehyde (TFTA). Experimental and computational results suggested that F–F interactions between the decorated tetrafluorobenzaldehyde groups and PFOA, as well as among the PFOA molecules themselves, would induce self-aggregation of PFOA molecules on the surfaces or in the pores of F–Cu–NH2BDC. This specific aggregation inhibited contact and electron transfer between F–Cu–NH2BDC and the electrolyte, resulting in a decrease in the inherent electrochemical Cu2+/Cu+ redox signal from F–Cu–NH2BDC. Based on this, an F–Cu–NH2BDC-based label- and probe-free PFOA electrochemical sensor was exploited with an excellent linear range from 5 pM to 500 μM and an extremely low detection limit of 3.54 pM, surpassing most currently reported electrochemical and nonelectrochemical PFAS sensors. This sensor also exhibited good stability, reproducibility, and anti-interference performance, enabling the accurate measurement of PFOA concentrations in actual commercial drinking water. These findings shed light on the design of PFAS sensors utilizing the F–F interaction as the working mechanism.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


