Postbiotics Made From Selected Lactic Acid Bacteria Improves Chronic Restraint Stress-Induced Anhedonia and Sleep Disorders
IF 4.5
2区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
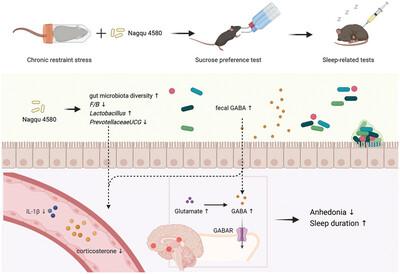
Sleep disorders have become one of the most prevalent neuropsychiatric disorders in recent years. This study aimed to investigate the effects of postbiotics derived from selected lactic acid bacteria on anhedonia and sleep disorders in chronic restraint stress (CRS)-induced mice, as well as their potential mechanisms. Mice were orally administered normal saline, low, medium, or high doses of postbiotics for 30 days, with CRS applied from days 1 to 21. The medium dose of postbiotics significantly increased the sucrose preference index, and the high dose of postbiotics significantly increased sleep duration. Postbiotic treatment effectively restored the diversity and composition of the gut microbiota to levels comparable to those observed in the vehicle (Veh) group. Furthermore, low and medium doses of postbiotics significantly reduced serum corticosterone levels, and medium and high doses significantly reduced serum IL-1β levels. Additionally, postbiotics administration significantly increased glutamate and GABA levels in both the prefrontal cortex and hypothalamus, as well as GABA levels in the feces. These results indicate that postbiotics alleviate CRS-induced anhedonia and sleep disorders in a dose-dependent manner. This effect may be mediated through the restoration of homeostasis in the MGB axis, HPA axis, inflammation pathways, and neurotransmitter balance.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Nutrition & Food Research
工程技术-食品科技
CiteScore
8.70
自引率
1.90%
发文量
250
审稿时长
1.7 months
期刊介绍:
Molecular Nutrition & Food Research is a primary research journal devoted to health, safety and all aspects of molecular nutrition such as nutritional biochemistry, nutrigenomics and metabolomics aiming to link the information arising from related disciplines:
Bioactivity: Nutritional and medical effects of food constituents including bioavailability and kinetics.
Immunology: Understanding the interactions of food and the immune system.
Microbiology: Food spoilage, food pathogens, chemical and physical approaches of fermented foods and novel microbial processes.
Chemistry: Isolation and analysis of bioactive food ingredients while considering environmental aspects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


