Mechanical alloyed FeCoNiMoM (M=Cr, cu) high-entropy alloy powders as electrocatalysts for oxygen evolution reaction
IF 9.6
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Here, micron-sized high-entropy alloy (HEA) electrocatalysts (FeCoNiMoCr, Cr-HEA; FeCoNiMoCu, Cu-HEA) with dual-phase heterostructures were fabricated by mechanical alloying and subsequently loaded onto nickel foam (NF) to form the working electrode, exhibiting excellent oxygen evolution reaction (OER) performance. Specifically, the Cr-HEA/NF exhibits an overpotential of 271 mV at current density of 10 mA/cm2 and a small Tafel slope of 69.1 mV/dec in 1 mol/L KOH solution, outperforming the performance of Cu-HEA/NF, commercial RuO2/NF and bare NF. HEA catalysts achieve outstanding long-term stability, as evidenced by chronopotentiometry (1 mol/L KOH for 48 h @10 mA/cm2 and 6 mol/L KOH at 85 °C for 100 h @500 mA/cm2) and chronoamperometry (1 mol/L KOH for 100 h @100 mA/cm2). The impressive OER activity and stability of Cr-HEA can be attributed to the highly heterogeneous nested interfaces between amorphous and metastable nanocrystals, as well as the in-situ formation of multiphase structures. Notably, both density functional theory calculations and experimental results demonstrate that the synergistic interactions among the metal active sites in HEA collectively regulate the adsorption and desorption of oxygen-containing intermediates, thereby enhancing the OER catalytic activity. Specifically, the Cr-HEA presents a lower Gibbs free energy change during the transformation from O∗ to OOH∗, resulting in a reduced overpotential.

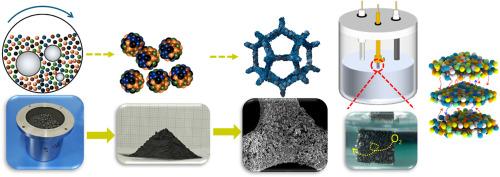
机械合金FeCoNiMoM (M=Cr, Cu)高熵合金粉末作为析氧反应的电催化剂
这里,微米级高熵合金(HEA)电催化剂(FeCoNiMoCr, Cr-HEA;采用机械合金化法制备了具有双相异质结构的FeCoNiMoCu、Cu-HEA,并将其加载到泡沫镍(NF)上形成工作电极,具有优异的析氧反应(OER)性能。其中,Cr-HEA/NF在电流密度为10 mA/cm2时的过电位为271 mV,在1 mol/L KOH溶液中,Tafel斜率较小,为69.1 mV/dec,优于Cu-HEA/NF、商用RuO2/NF和裸NF。HEA催化剂具有出色的长期稳定性,时间电位测定(1 mol/L KOH, 48小时@10 mA/cm2, 6 mol/L KOH, 85°C, 100小时@500 mA/cm2)和时间电流测定(1 mol/L KOH, 100小时@100 mA/cm2)证明了这一点。Cr-HEA令人印象深刻的OER活性和稳定性可归因于非晶和亚稳纳米晶体之间的高度非均相嵌套界面,以及多相结构的原位形成。值得注意的是,密度泛函理论计算和实验结果都表明,HEA中金属活性位点之间的协同作用共同调节了含氧中间体的吸附和解吸,从而提高了OER的催化活性。具体来说,Cr-HEA在O*到OOH*的转变过程中呈现出较低的吉布斯自由能变化,导致过电位降低。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materiomics
Materials Science-Metals and Alloys
CiteScore
14.30
自引率
6.40%
发文量
331
审稿时长
37 days
期刊介绍:
The Journal of Materiomics is a peer-reviewed open-access journal that aims to serve as a forum for the continuous dissemination of research within the field of materials science. It particularly emphasizes systematic studies on the relationships between composition, processing, structure, property, and performance of advanced materials. The journal is supported by the Chinese Ceramic Society and is indexed in SCIE and Scopus. It is commonly referred to as J Materiomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


