Molecular Mechanism of λ-Cyhalothrin Detoxification by a Delta-Class Glutathione S-Transferase (PxGSTD3) from Plutella xylostella
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The diamondback moth (Plutella xylostella) exhibits significant resistance to commonly used insecticides including λ-cyhalothrin. Delta-class glutathione S-transferases (GSTs) are crucial detoxification enzymes involved in insecticide detoxification and resistance. We demonstrate that PxGSTD3 is associated with the resistance to λ-cyhalothrin and contributes to λ-cyhalothrin detoxification. The transcription of PxGSTD3 was rapidly upregulated in response to λ-cyhalothrin exposure, and the recombinant protein exhibited significant metabolic activity against λ-cyhalothrin. Further investigation using computer-aided drug design revealed the binding and metabolic mechanism of PxGSTD3 toward λ-cyhalothrin. The results showed that λ-cyhalothrin binds to an active pocket through noncovalent interactions such as hydrogen bonds, π–π stacking, and hydrophobic interactions. Residues Arg36, Tyr115, and Phe119 were found to have a critical impact on the binding and metabolism of λ-cyhalothrin by PxGSTD3. These findings provide valuable insights into the metabolic role of GST in detoxifying insecticides and offer theoretical guidance for the design of novel pyrethroid-based insecticides.
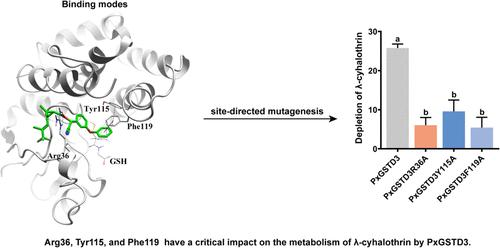
小菜菜δ级谷胱甘肽s -转移酶(PxGSTD3)解毒λ-氯氟氰菊酯的分子机制
小菜蛾(Plutella xylostella)对λ-氯氟氰菊酯等常用杀虫剂具有显著的抗性。Delta-class谷胱甘肽s -转移酶(GSTs)是参与杀虫剂解毒和抗性的重要解毒酶。我们证明了PxGSTD3与λ-氯氟氰菊酯抗性有关,并有助于λ-氯氟氰菊酯解毒。PxGSTD3在暴露于λ-氯氟氰菊酯后转录迅速上调,重组蛋白对λ-氯氟氰菊酯表现出显著的代谢活性。计算机辅助药物设计进一步揭示了PxGSTD3对λ-氯氟氰菊酯的结合和代谢机制。结果表明,λ-氯氟氰菊酯通过氢键、π -π堆叠和疏水等非共价相互作用与活性袋结合。发现残基Arg36、Tyr115和Phe119对PxGSTD3结合和代谢λ-氯氟氰菊酯具有关键影响。这些发现对GST在杀虫剂解毒中的代谢作用提供了有价值的见解,并为新型拟除虫菊酯类杀虫剂的设计提供了理论指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


