Design, Synthesis, and Biological Evaluation of Asymmetrical Disulfides Based on Garlic Extract as Pseudomonas aeruginosa pqs Quorum Sensing Inhibitors
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
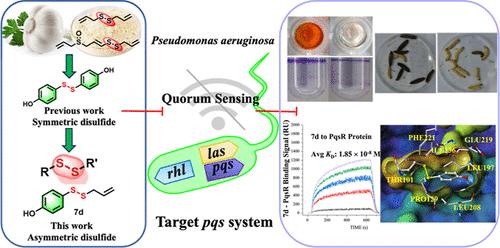
Pseudomonas aeruginosa is a widely encountered bacterium linked to the deterioration of food products and represents a notable concern for public health safety. Disulfides serve as significant pharmacologically active scaffolds exhibiting antibacterial, antiviral, and anticancer properties; however, reports on their activity as quorum sensing inhibitors (QSIs) against P. aeruginosa are limited. In our work, asymmetrical disulfides were designed and synthesized, utilizing natural products, such as allicin, ajoene, diallyl disulfide (DADS), hordenine, and cinnamic acid, as lead compounds. By screening for lasB, rhlA, and pqsA promoter activity, two highly effective QSIs were identified. Compounds 7d and 4c show effectiveness in reducing the synthesis of different virulence factors, the creation of biofilms, and movement capabilities. Subsequent validation using the Galleria mellonella larvae model confirmed their robust in vivo efficacy. Moreover, their combination with antibiotics markedly augmented the antibacterial activity. Mechanism studies employed by transcriptome analysis, quantitative reverse transcription-PCR (qRT-PCR), surface plasmon resonance, and molecular docking demonstrate that compound 7d disrupts the quorum sensing system by interacting with PqsR. These findings suggest that our disulfide derivatives hold promise for treating P. aeruginosa infections.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


