Amphiphilic food polypeptides via moderate enzymatic hydrolysis of chickpea proteins: Bioprocessing, properties, and molecular mechanism
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Plant proteins are a promising source for producing amphiphilic polypeptides with tailored techno-functional properties to be used in various food applications, such as fat replacers. This study investigated the effects of moderate enzymatic hydrolysis on amphiphilic polypeptide generation, by understanding the relationship of bioprocess – protein structure – functionality – amphiphilicity mechanism. Compared to non-specific protease alcalase, the specific protease trypsin catalyzed the production of polypeptides with higher surface hydrophobicity and relatively high molecular weight. Trypsin-produced polypeptides exhibited significantly higher water and oil holding capacities, foaming capacities, and emulsification than alcalase-produced counterparts. Furthermore, polypeptide sequences were obtained from proteomics and used to analyze amphiphilicity using Grand Average of Hydropathy (GRAVY) scores and hydropathy plots. Trypsin produced high number of amphiphilic polypeptides with balanced hydrophilic and hydrophobic regions. Molecular dynamics (MD) simulations of selected amphiphilic polypeptides in water-oleic acid systems suggested strong hydrophobic interactions with oleic acid and stable conformations in the interface.
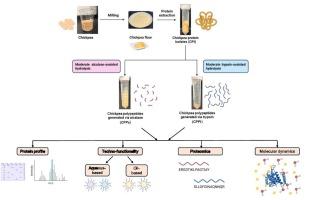
鹰嘴豆蛋白经适度酶解的两亲性食品多肽:生物加工、性质和分子机制
植物蛋白是生产具有特定技术功能特性的两亲性多肽的有前途的来源,可用于各种食品应用,如脂肪替代品。本研究通过了解生物过程-蛋白质结构-功能-两亲性机理的关系,探讨了适度酶解对两亲性多肽生成的影响。与非特异性蛋白酶alcalase相比,特异性蛋白酶胰蛋白酶催化的多肽具有更高的表面疏水性和相对较高的分子量。胰蛋白酶产生的多肽比碱性蛋白酶产生的多肽具有更高的持水和持油能力、起泡能力和乳化能力。此外,从蛋白质组学中获得多肽序列,并使用大平均水亲性评分和水亲性图来分析两亲性。胰蛋白酶产生大量亲疏水区平衡的两亲多肽。水-油酸体系中两亲性多肽的分子动力学(MD)模拟表明,两亲性多肽与油酸有很强的疏水相互作用,界面构象稳定。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


