Microfluidic Replication and Phenotypic Profiling of Extracellular Vesicles from the Tumor Microenvironment Using Dual-Switch Aptamer Logic Gates
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
The phenotypic profiling of extracellular vesicles (EVs) within the tumor microenvironment (TME) provides critical insights into the intercellular communication mechanisms of EVs underlying tumor physiology. However, conventional methods typically isolate EVs from the extracellular space through tissue fragmentation, which compromises tissue viability, and neglects the spatial organization of the tissue and the dynamic nature of EV secretion. Herein, we introduce an innovative microfluidic platform to cultivate intact tumor tissues while preserving their spatial architecture and facilitating natural EV secretion. This system enables the direct replication of EVs onto the chip for high-fidelity phenotypic analysis. Utilizing a combinatorial-aptamer-induced dual-switch logic gate methodology, this approach allows for the precise subtyping of EVs derived from both tumor cells and immune cells within the TME. Specifically, aptamers targeting EpCAM and PD-L1, along with the connector probe, were employed to induce a dual-switch signal to identify distinct EV populations. This strategy enables noninvasive, real-time capture and phenotypic profiling of EVs directly within the microfluidic environment. Furthermore, our findings indicate that immunotherapy with PD-1 antibodies significantly enhances the secretion of EVs by immune cells within the TME, underscoring the potential role of EVs as mediators of therapeutic responses. Overall, we have developed a robust, noninvasive method for the phenotypic profiling of EVs in the TME, offering a powerful tool for investigating the biological functions and implications of EVs in tumor pathophysiology.
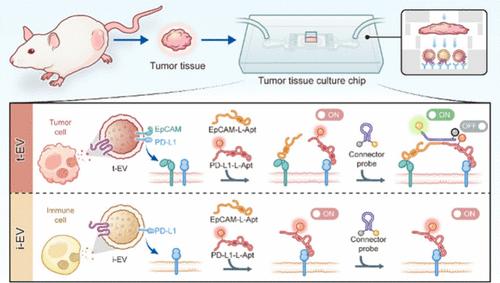
使用双开关适体逻辑门的肿瘤微环境细胞外囊泡的微流控复制和表型分析
肿瘤微环境(TME)中细胞外囊泡(EVs)的表型分析为EVs在肿瘤生理学基础上的细胞间通讯机制提供了重要见解。然而,传统的方法通常通过组织破碎从细胞外空间分离出EV,这损害了组织的活力,忽视了组织的空间组织和EV分泌的动态性。在此,我们引入了一种创新的微流控平台来培养完整的肿瘤组织,同时保留其空间结构并促进EV的自然分泌。该系统能够将ev直接复制到芯片上,以进行高保真表型分析。利用组合适体诱导的双开关逻辑门方法,该方法允许对来自肿瘤细胞和TME内免疫细胞的ev进行精确的分型。具体来说,针对EpCAM和PD-L1的适体,以及连接器探针,被用来诱导双开关信号来识别不同的EV群体。该策略可以在微流体环境中直接对电动汽车进行无创、实时捕获和表型分析。此外,我们的研究结果表明,PD-1抗体的免疫治疗显著增强了TME内免疫细胞的ev分泌,强调了ev作为治疗反应介质的潜在作用。总的来说,我们已经开发了一种强大的、无创的方法来分析TME中EVs的表型,为研究EVs在肿瘤病理生理中的生物学功能和意义提供了一个强大的工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


