A Double-Locked ESIPT-AIE Fluorescent Probe Detects Esterase with Highly Matched Response Kinetics
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
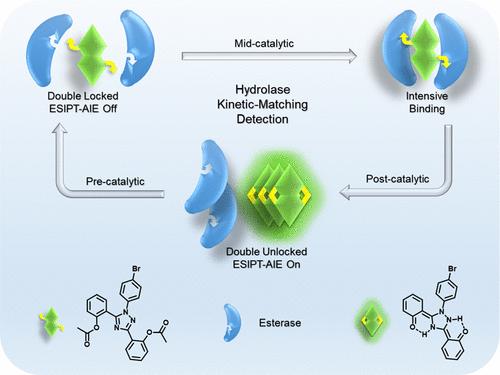
Hydrolyases play an irreplaceable role in complex biological processes, and their dysfunction is a cause of many human diseases. Advanced activatable in situ fluorescence detection methods offer high-resolution spatiotemporal analysis, aiding in the dissection of the complex biological roles of hydrolases. However, current strategies typically focus on only specific stages of enzyme-probe interactions, leading to suboptimal imaging fidelity and sometimes erroneous detection results. Addressing this, we developed a double-locked “Excited State Intramolecular Proton Transfer-Aggregation Induced Emission (ESIPT-AIE)” fluorescent probe (Br-3N-2Et) that matches the entire enzymatic response kinetics for enzyme activity detection. We validated the probe’s mechanism by enhancing pre-reaction recognition through double unlockable recognition sites, thereby reducing basal fluorescence (Φ = 0.0183) and increasing resistance to interference signals. Subsequently, the ESIPT fluorophore with multiple hydrogen bonds enhanced the affinity for the hydrolase catalytic site, improving binding kinetics and exhibiting a significant Stokes shift (188 nm). The realization of the ESIPT-AIE dual-emission mechanism facilitated rapid efflux of the fluorophore from the catalytic site and subsequent in situ fluorescence signal enhancement (132.2-fold). This new probe achieved regional differential detection of esterase activity in HepG2 cells and endometrial cancer tissues. Thus, this work paves the way for the development of integrated, multimechanism platforms for hydrolase activity fluorescence sensing and imaging in complex biochemical contexts.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


