Saltiness Enhancement of Soy Peptides by Modulating Amiloride-Insensitive Salt-Responsive Cells and Interacting with Cell Membranes
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Saltiness-enhancing peptides hold great potential for salt reduction in the food industry. This study investigated the saltiness-enhancing mechanism of soy peptides E (EDEGEQPRPF), DG (DEGEQPRPFP), and 9AA (DEGEQPRPF), focusing on their interactions with amiloride-insensitive taste cells and cell membranes. Sensory evaluation showed that adding E and DG (0.4 mg/mL) to 50 mM NaCl increased perceived saltiness to 61.4 and 54.78 mM NaCl, while 9AA had no effect. Calcium imaging of taste organoids highlighted the role of Cl– in the amiloride-insensitive pathway. Peptide E enhanced the response of amiloride-insensitive salt-responsive cells by 35.19%, while DG and 9AA did not. Single-cell RNA sequencing revealed no functional ENaC heterotrimer and high Tmc4 expression in all types of taste cells, while Trpv1 was found in only one circumvallate papilla (CV) taste cell. E and DG form more stable bonds with TMC4 via hydrogen bonds and water bridges compared to 9AA, as evidenced by molecular dynamics simulations. Negatively charged peptide E, with an α-helical-like structure, adsorbed onto liposomes more than DG and 9AA due to its N-terminal Glu, suggesting E may indirectly modulate taste receptor function by altering membrane potential. These findings provide insights into the structure–function relationship of saltiness-enhancing peptides.
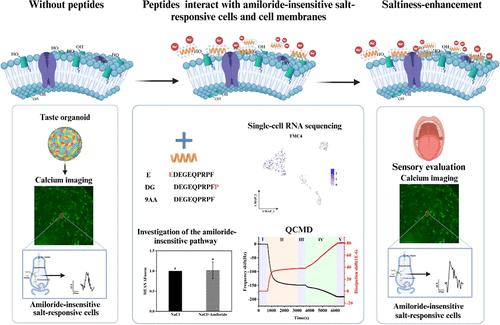
通过调节阿米洛利不敏感的盐反应细胞和与细胞膜相互作用提高大豆肽的咸味
增盐肽在食品工业中具有巨大的减盐潜力。本研究研究了大豆肽E (EDEGEQPRPF)、DG (DEGEQPRPFP)和9AA (DEGEQPRPF)的增咸机制,重点研究了它们与氨酰胺不敏感味觉细胞和细胞膜的相互作用。感官评价结果表明,在50 mM NaCl中添加0.4 mg/mL的E和DG可使盐的感觉咸度分别达到61.4和54.78 mM NaCl,而9AA对盐的感觉咸度无影响。味觉类器官的钙显像强调了Cl -在阿米洛利不敏感通路中的作用。多肽E可使阿米洛利不敏感的盐反应细胞的反应增强35.19%,而DG和9AA则无此作用。单细胞RNA测序结果显示,在所有类型的味觉细胞中均未发现ENaC异源三聚体,Tmc4高表达,而Trpv1仅在一个环状乳头(CV)味觉细胞中发现。分子动力学模拟结果表明,与9AA相比,E和DG通过氢键和水桥与TMC4形成更稳定的键。带负电荷的肽E具有α-螺旋状结构,由于其n端Glu,比DG和9AA更多地吸附在脂质体上,表明E可能通过改变膜电位间接调节味觉受体功能。这些发现为盐度增强肽的结构-功能关系提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


