Three systems of circuit formation: assembly, updating and tuning
IF 26.7
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Understanding the relationship between genotype and neuronal circuit phenotype necessitates an integrated view of genetics, development, plasticity and learning. Challenging the prevailing notion that emphasizes learning and plasticity as primary drivers of circuit assembly, in this Perspective, we delineate a tripartite framework to clarify the respective roles that learning and plasticity might have in this process. In the first part of the framework, which we term System One, neural circuits are established purely through genetically driven algorithms, in which spike timing-dependent plasticity serves no instructive role. We propose that these circuits equip the animal with sufficient skill and knowledge to successfully engage the world. Next, System Two is governed by rare but critical ‘single-shot learning’ events, which occur in response to survival situations and prompt rapid synaptic reconfiguration. Such events serve as crucial updates to the existing hardwired knowledge base of an organism. Finally, System Three is characterized by a perpetual state of synaptic recalibration, involving continual plasticity for circuit stabilization and fine-tuning. By outlining the definitions and roles of these three core systems, our framework aims to resolve existing ambiguities related to and enrich our understanding of neural circuit formation. In this Perspective, Barabási, Ferreira Castro and Engert challenge the notion that learning and plasticity primarily drive the assembly of neural circuits. They present a tripartite framework for how neural circuits form, outlining the relative contributions of developmental, associative learning and tuning-based factors to this process and knowledge acquisition.

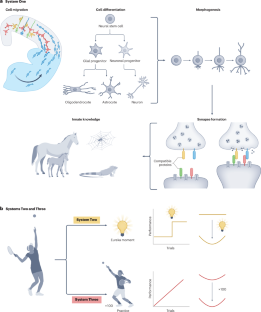
电路形成的三个系统:装配、更新和调谐
理解基因型和神经元回路表型之间的关系需要从遗传学、发育、可塑性和学习等方面综合考虑。挑战强调学习和可塑性是电路组装的主要驱动因素的流行观念,在这个观点中,我们描绘了一个三方框架,以阐明学习和可塑性在这一过程中可能发挥的各自作用。在框架的第一部分,我们称之为系统一,神经回路纯粹是通过遗传驱动算法建立的,其中峰值时间依赖的可塑性没有指导作用。我们认为这些回路使动物具备了足够的技能和知识来成功地与世界交往。其次,系统二由罕见但关键的“单次学习”事件控制,这些事件发生在对生存情况的反应中,并促使突触快速重构。这些事件是对生物体现有的固有知识基础的重要更新。最后,系统三的特点是突触重新校准的永久状态,涉及电路稳定和微调的持续可塑性。通过概述这三个核心系统的定义和作用,我们的框架旨在解决与神经回路形成相关的现有歧义,并丰富我们对神经回路形成的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Neuroscience
NEUROSCIENCES-
自引率
0.60%
发文量
104
期刊介绍:
Nature Reviews Neuroscience is a multidisciplinary journal that covers various fields within neuroscience, aiming to offer a comprehensive understanding of the structure and function of the central nervous system. Advances in molecular, developmental, and cognitive neuroscience, facilitated by powerful experimental techniques and theoretical approaches, have made enduring neurobiological questions more accessible. Nature Reviews Neuroscience serves as a reliable and accessible resource, addressing the breadth and depth of modern neuroscience. It acts as an authoritative and engaging reference for scientists interested in all aspects of neuroscience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


