CRISPR-Cas12a/Cas13a Multiplex Bioassay for ctDNA and miRNA by Mass Spectrometry
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
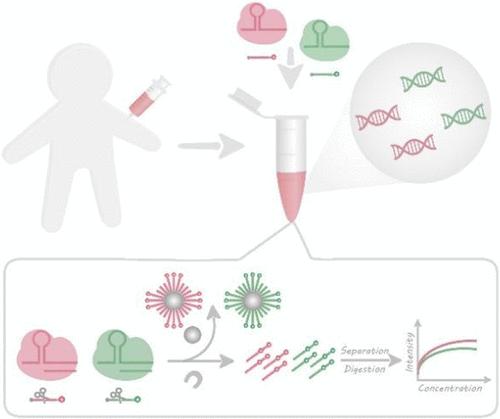
The CRISPR-Cas system, particularly CRISPR-Cas12a and CRISPR-Cas13a, has been widely utilized in constructing various biosensors due to their “trans-cleavage” ability as a means of signal amplification. However, this universal “trans-cleavage” characteristic also presents a challenge for realizing CRISPR-Cas multiplexed bioanalysis. Besides, potential signal cascading interference and complicated design are notable obstacles in CRISPR-Cas multiplexed bioanalysis. Herein, we propose a mass spectrometry method that leverages the CRISPR-Cas12a/13a system to achieve simultaneous detection of ctDNA and miRNA. Based on the properties of the CRISPR-Cas12a/13a system, two types of nanoparticle reporter probes have been engineered, using cancer-related biomarkers ctDNA and miR-21 as our model analytes. The nanoparticle tags, which intrinsically incorporated millions of detectable atoms, combined with the CRISPR-Cas12a/Cas13a system’s “trans-cleavage” ability, allow the proposed mass spectrometry strategy to achieve fmol-level detection limits without any nucleic acid amplification procedures. The assay was successfully applied to human serum samples, demonstrating its potential for early disease diagnosis and progression tracking.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


